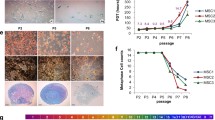
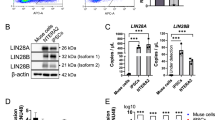
Abstract
Stem cell senescence is considered deleterious because it may impair tissue renewal and function. On the other hand, senescence may arrest the uncontrolled growth of transformed stem cells and protect organisms from cancer. This double function of senescence is strictly linked to the activity of genes that the control cell cycle such as the retinoblastoma proteins RB1, RB2/P130, and P107. We took advantage of the RNA interference technique to analyze the role of these proteins in the biology of mesenchymal stem cells (MSC). Cells lacking RB1 were prone to DNA damage. They showed elevated levels of p53 and p21cip1 and increased regulation of RB2/P130 and P107 expression. These cells gradually adopted a senescent phenotype with impairment of self-renewal properties. No significant modification of cell growth was observed as it occurs in other cell types or systems. In cells with silenced RB2/P130, we detected a reduction of DNA damage along with a higher proliferation rate, an increase in clonogenic ability, and the diminution of apoptosis and senescence. Cells with silenced RB2/P130 were cultivated for extended periods of time without adopting a transformed phenotype. Of note, acute lowering of P107 did not induce relevant changes in the in vitro behavior of MSC. We also analyzed cell commitment and the osteo-chondro-adipogenic differentiation process of clones derived by MSC cultures. In all clones obtained from cells with silenced retinoblastoma genes, we observed a reduction in the ability to differentiate compared with the control clones. In summary, our data show evidence that the silencing of the expression of RB1 or RB2/P130 is not compensated by other gene family members, and this profoundly affects MSC functions.





Similar content being viewed by others
References
Collado M, Blasco MA, Serrano M (2007) Cellular senescence in cancer and aging. Cell 130(2):223–233 [pii: S0092-8674(07)00890-2]
Galderisi U, Cipollaro M, Giordano A (2006) The retinoblastoma gene is involved in multiple aspects of stem cell biology. Oncogene 25(38):5250–5256
Galderisi U, Jori FP, Giordano A (2003) Cell cycle regulation and neural differentiation. Oncogene 22(33):5208–5219
Claudio PP, Howard CM, Baldi A, De Luca A, Fu Y, Condorelli G, Sun Y, Colburn N, Calabretta B, Giordano A (1994) p130/pRb2 has growth suppressive properties similar to yet distinctive from those of retinoblastoma family members pRb and p107. Cancer Res 54(21):5556–5560
Herrera RE, Sah VP, Williams BO, Makela TP, Weinberg RA, Jacks T (1996) Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol Cell Biol 16(5):2402–2407
MacPherson D, Sage J, Crowley D, Trumpp A, Bronson RT, Jacks T (2003) Conditional mutation of Rb causes cell cycle defects without apoptosis in the central nervous system. Mol Cell Biol 23(3):1044–1053
MacPherson D, Sage J, Kim T, Ho D, McLaughlin ME, Jacks T (2004) Cell type-specific effects of Rb deletion in the murine retina. Genes Dev 18(14):1681–1694
Haigis K, Sage J, Glickman J, Shafer S, Jacks T (2006) The related retinoblastoma (pRb) and p130 proteins cooperate to regulate homeostasis in the intestinal epithelium. J Biol Chem 281(1):638–647
Beyer Nardi N, da Silva Meirelles L (2006) Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol 174:249–282
Bianco P, Riminucci M, Gronthos S, Gehron Robey P (2001) Bone marrow stromal stem cells: nature, biology and potential applications. Stem Cells 19:180–192
Muller-Sieburg CE, Deryugina E (1995) The stromal cells’ guide to the stem cell universe. Stem Cells 13(5):477–486
Beausejour C (2007) Bone marrow-derived cells: the influence of aging and cellular senescence. Handb Exp Pharmacol 180:67–88
Pelicci PG (2004) Do tumor-suppressive mechanisms contribute to organism aging by inducing stem cell senescence? J Clin Invest 113(1):4–7
Sethe S, Scutt A, Stolzing A (2006) Aging of mesenchymal stem cells. Ageing Res Rev 5(1):91–116
Giordano A, Galderisi U, Marino IR (2007) From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol 211(1):27–35
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317
Nakamura S, Yamada Y, Baba S, Kato H, Kogami H, Takao M, Matsumoto N, Ueda M (2008) Culture medium study of human mesenchymal stem cells for practical use of tissue engineering and regenerative medicine. Biomed Mater Eng 18(3):129–136
Pochampally R (2008) Colony-forming unit assays for MSCs. Methods Mol Biol 449:83–91
Muraglia A, Cancedda R, Quarto R (2000) Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci 113(Pt 7):1161–1166
Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O (2009) Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4(12):1798–1806
Gary RK, Kindell SM (2005) Quantitative assay of senescence-associated beta-galactosidase activity in mammalian cell extracts. Anal Biochem 343(2):329–334
Galderisi U, Di Bernardo G, Cipollaro M, Peluso G, Cascino A, Cotrufo R, Melone MA (1999) Differentiation and apoptosis of neuroblastoma cells: role of N-myc gene product. J Cell Biochem 73(1):97–105
Jori FP, Melone MA, Napolitano MA, Cipollaro M, Cascino A, Giordano A, Galderisi U (2005) RB and RB2/p130 genes demonstrate both specific and overlapping functions during the early steps of in vitro neural differentiation of marrow stromal stem cells. Cell Death Differ 12(1):65–77
Walen KH (2006) Human diploid fibroblast cells in senescence; cycling through polyploidy to mitotic cells. In Vitro Cell Dev Biol Anim 42(7):216–224
Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW (2005) DNA repair, genome stability, and aging. Cell 120(4):497–512
Roos WP, Kaina B (2006) DNA damage-induced cell death by apoptosis. Trends Mol Med 12(9):440–450
Cleaver JE, Crowley E (2002) UV damage, DNA repair and skin carcinogenesis. Front Biosci 7:d1024–d1043
D’Errico M, Parlanti E, Dogliotti E (2008) Mechanism of oxidative DNA damage repair and relevance to human pathology. Mutat Res 659(1–2):4–14
Kurz EU, Douglas P, Lees-Miller SP (2004) Doxorubicin activates ATM-dependent phosphorylation of multiple downstream targets in part through the generation of reactive oxygen species. J Biol Chem 279(51):53272–53281
Nitiss JL (2002) DNA topoisomerases in cancer chemotherapy: using enzymes to generate selective DNA damage. Curr Opin Investig Drugs 3(10):1512–1516
Zuckerman V, Wolyniec K, Sionov RV, Haupt S, Haupt Y (2009) Tumour suppression by p53: the importance of apoptosis and cellular senescence. J Pathol 219(1):3–15
Campisi J, d’Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8(9):729–740
Attema JL, Pronk CJ, Norddahl GL, Nygren JM, Bryder D (2009) Hematopoietic stem cell ageing is uncoupled from p16 INK4A-mediated senescence. Oncogene 28(22):2238–2243
Mirzayans R, Andrais B, Scott A, Paterson MC, Murray D (2010) Single-cell analysis of p16(INK4a) and p21(WAF1) expression suggests distinct mechanisms of senescence in normal human and Li-Fraumeni syndrome fibroblasts. J Cell Physiol 223(1):57–67
Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F (2007) Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 67(19):9142–9149
Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA (2006) Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 99(5):1285–1297
Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lonning PE, Bjerkvig R, Schichor C (2009) Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res 69(13):5331–5339
Giacinti C, Giordano A (2006) RB and cell cycle progression. Oncogene 25(38):5220–5227
Paggi MG, Giordano A (2001) Who is the boss in the retinoblastoma family? The point of view of Rb2/p130, the little brother. Cancer Res 61(12):4651–4654
Chicas A, Wang X, Zhang C, McCurrach M, Zhao Z, Mert O, Dickins RA, Narita M, Zhang M, Lowe SW (2010) Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell 17(4):376–387
Laurie N, Mohan A, McEvoy J, Reed D, Zhang J, Schweers B, Ajioka I, Valentine V, Johnson D, Ellison D, Dyer MA (2009) Changes in retinoblastoma cell adhesion associated with optic nerve invasion. Mol Cell Biol 29(23):6268–6282
Haneline LS (2008) Redox regulation of stem and progenitor cells. Antioxid Redox Signal 10(11):1849–1852
Zanichelli F, Capasso S, Cipollaro M, Pagnotta E, Carteni M, Casale F, Iori R, Galderisi U (2012) Dose-dependent effects of R-sulforaphane isothiocyanate on the biology of human mesenchymal stem cells, at dietary amounts, it promotes cell proliferation and reduces senescence and apoptosis, while at anti-cancer drug doses, it has a cytotoxic effect. Age (Dordr) 34(2):281–293
Zanichelli F, Capasso S, Di Bernardo G, Cipollaro M, Pagnotta E, Carteni M, Casale F, Iori R, Giordano A, Galderisi U (2012) Low concentrations of isothiocyanates protect mesenchymal stem cells from oxidative injuries, while high concentrations exacerbate DNA damage. Apoptosis 17(9):964–974
Guo Y, Einhorn L, Kelley M, Hirota K, Yodoi J, Reinbold R, Scholer H, Ramsey H, Hromas R (2004) Redox regulation of the embryonic stem cell transcription factor oct-4 by thioredoxin. Stem Cells 22(3):259–264
Kuilman T, Peeper DS (2009) Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9(2):81–94
Nandy C, Mrazek J, Stoiber H, Grasser FA, Huttenhofer A, Polacek N (2009) Epstein–Barr virus-induced expression of a novel human vault RNA. J Mol Biol 388(4):776–784
Montanaro L, Trere D, Derenzini M (2008) Nucleolus, ribosomes, and cancer. Am J Pathol 173(2):301–310
White RJ (1997) Regulation of RNA polymerases I and III by the retinoblastoma protein: a mechanism for growth control? Trends Biochem Sci 22(3):77–80
Helmbold H, Galderisi U, Bohn W (2011) The switch from Rb1/p105 to Rb2/p130 in DNA damage and cellular senescence. J Cell Physiol 227(2):508–513
Takahashi P, Polson A, Reisman D (2011) Elevated transcription of the p53 gene in early S-phase leads to a rapid DNA damage response during S-phase of the cell cycle. Apoptosis 16(9):950–958
Grimmler M, Wang Y, Mund T, Cilensek Z, Keidel EM, Waddell MB, Jakel H, Kullmann M, Kriwacki RW, Hengst L (2007) Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128(2):269–280
Kapic A, Helmbold H, Reimer R, Klotzsche O, Deppert W, Bohn W (2006) Cooperation between p53 and p130(Rb2) in induction of cellular senescence. Cell Death Differ 13(2):324–334
Odell A, Askham J, Whibley C, Hollstein M (2010) How to become immortal: let MEFs count the ways. Aging (Albany NY) 2(3):160–165
Helmbold H, Deppert W, Bohn W (2006) Regulation of cellular senescence by Rb2/p130. Oncogene 25(38):5257–5262
Helmbold H, Komm N, Deppert W, Bohn W (2009) Rb2/p130 is the dominating pocket protein in the p53–p21 DNA damage response pathway leading to senescence. Oncogene 28(39):3456–3467
Hurford RK Jr, Cobrinik D, Lee MH, Dyson N (1997) pRB and p107/p130 are required for the regulated expression of different sets of E2F-responsive genes. Genes Dev 11(11):1447–1463
Jackson JG, Pereira-Smith OM (2006) Primary and compensatory roles for RB family members at cell cycle gene promoters that are deacetylated and downregulated in doxorubicin-induced senescence of breast cancer cells. Mol Cell Biol 26(7):2501–2510
Zhu L (2005) Tumour suppressor retinoblastoma protein RB: a transcriptional regulator. Eur J Cancer 41(16):2415–2427
Hansen JB, te Riele H, Kristiansen K (2004) Novel function of the retinoblastoma protein in fat: regulation of white versus brown adipocyte differentiation. Cell Cycle 3(6):774–778
Richon VM, Lyle RE, McGehee RE Jr (1997) Regulation and expression of retinoblastoma proteins p107 and p130 during 3T3-L1 adipocyte differentiation. J Biol Chem 272(15):10117–10124
Higgins C, Chatterjee S, Cherington V (1996) The block of adipocyte differentiation by a C-terminally truncated, but not by full-length, simian virus 40 large tumor antigen is dependent on an intact retinoblastoma susceptibility protein family binding domain. J Virol 70(2):745–752
Chen PL, Riley DJ, Chen Y, Lee WH (1996) Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev 10(21):2794–2804
Fajas L, Egler V, Reiter R, Hansen J, Kristiansen K, Debril MB, Miard S, Auwerx J (2002) The retinoblastoma-histone deacetylase 3 complex inhibits PPARgamma and adipocyte differentiation. Dev Cell 3(6):903–910
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2012_1224_MOESM3_ESM.tif
Supplemental File 3. Long-term culture of shR2 cells. A: Contrast phase microscopic field of shR2 cells at different time points. B: Polyacrylamide gel electrophoresis of TRAP assay products obtained from shR2-MSCs at different time points. The image shows the PCR-amplified products indicating telomerase activity. The assay measures the enzymatic activity of telomerase. In the first step of the reaction, active telomerase in cell extracts adds a varying number of telomeric repeats (TTAGGG) onto the 3′ end of a substrate oligonucleotide. Next, PCR is used to amplify the extended products. Products are then electrophoresed on a polyacrylamide gel (see details of the method in Supplementary File 1). C: Differentiation potential of shR2 cells that were cultivated for 12 months. Cells were induced to differentiate into adipocytes, osteocytes, and chondrocytes, as described in the Materials and methods section. The picture on the left shows adipocytes stained with Oil Red. In the middle, differentiated chondrocytes were revealed by staining with Fast Green. On the right, differentiated osteocytes were revealed by staining with Alizarin Red. (TIFF 4381 kb)
18_2012_1224_MOESM4_ESM.tif
Supplemental File 4. Annexin assay. The micrographs show a higher magnification of figure 3A, with representative fields of cells stained with Annexin V (green). Nuclei were counterstained with Hoechst 33342 (blue). Arrows indicate Annexin-positive cells. (TIFF 13091 kb)
18_2012_1224_MOESM5_ESM.tif
Supplemental File 5. Senescence assay. The micrographs show a higher magnification of figure 3B, with representative fields acid beta-galactosidase (blue) in cells with silenced retinoblastoma proteins. (TIFF 15667 kb)
18_2012_1224_MOESM6_ESM.tif
Supplemental File 6. H2AX staining. The micrographs show a higher magnification of figure 3C, with representative fields of cells stained with anti-H2AX (green) and Hoechst 33342 (blue). Arrows indicate double-stained cells. (TIFF 3460 kb)
18_2012_1224_MOESM7_ESM.tif
Supplemental File 7. Anti-8-oxo-dG staining. The micrograph shows a higher magnification of figure 3D, with representative fields of cells stained with anti-8-oxo-dG (green) and Hoechst 33342 (blue). Arrows indicate double-stained cells. (TIFF 25240 kb)
18_2012_1224_MOESM10_ESM.tif
Supplemental File 10. Real-time RT-PCR validation of microarray data. We selected some genes that showed significant changes in their expression according to microarray analysis to validate microarray data by real-time RT-PCR. In cells expressing either shR1, shR2, sh107, or shCTRL, the mRNA levels of genes under analysis were normalized with respect to HPRT, chosen as an internal control. The histogram shows the ratio of gene expression between treated and control cells (shCTRL). The mean expression values (±SD, n = 3; * p < 0.05) of each gene are presented. (TIFF 95 kb)
Rights and permissions
About this article
Cite this article
Alessio, N., Bohn, W., Rauchberger, V. et al. Silencing of RB1 but not of RB2/P130 induces cellular senescence and impairs the differentiation potential of human mesenchymal stem cells. Cell. Mol. Life Sci. 70, 1637–1651 (2013). https://doi.org/10.1007/s00018-012-1224-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1224-x