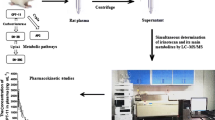
Abstract
In this paper, a fast method for the detection of irinotecan (CPT-11) in plasma samples was investigated. CPT-11 is widely used in a number of chemotherapeutic treatments of several solid tumors. The method is based on the combination of a solid phase extraction and an electrochemical detection step. The extraction of CPT-11 from plasma was performed using solid phase extraction (SPE) columns and acetonitrile as eluent. The procedure included also a cleaning step to eliminate interference due to plasma endogenous compounds and the co-therapeutics 5-fluoroacil (5-FU) and folinic acid (FA). The latter are administered together with CPT-11 in the FOLFIRI regimen. The detection of CPT-11 was performed by differential pulse voltammetry at a glassy carbon electrode (GCE) in basified acetonitrile media. Under these conditions, a well-defined peak due to the oxidation of the tertiary ammine end of CPT-11, also free from interference due to main metabolites, was obtained. Calibration plots showed a good linear response with limit of detection and quantification of 1.10 × 10−7 and 3.74 × 10−7 M, respectively. The suitability of the method proposed here for clinical applications was verified by determining the concentration of CPT-11 in plasma samples of an oncological patient, collected after 30 and 180 min from the infusion of the drug.

Graphical abstract







Similar content being viewed by others
References
Matsuzaki T, Yokokura T, Mutai M, Tsuruo T. Inhibition of spontaneous and experimental metastasis by a new derivative of camptothecin, CPT-11, in mice. Cancer Chemother Pharmacol. 1988;21:308–12.
Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187–91.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42.
de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S. Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet. 2018;57:1229–54.
Shafiei M, Yoon R, McLachlan A, Boddy A, Beale P, ;Prunella Blinman P. Pharmacokinetics of anticancer drugs used in treatment of older adults with colorectal cancer: a systematic review, Ther Drug Monit 2019; 41:553–560.
Kümler I, Balslev E, Stenvang J, Brünner N, Ejlertsen B, Jakobsen EH, et al. Two open-label, single arm, nonrandomized phase II studies of irinotecan for the treatment of metastatic breast cancer in patients with increased copy number of the topoisomerase I gene. BMC Cancer. 2019;19:573.
Mathijssen RHJ, Van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G. Sparreboom A Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res. 2001;7:2182–94.
Tamargo J, Le Heuzey JY, Mabo P. Narrow therapeutic index drugs: a clinical pharmacological consideration to flecainide. Eur J Clin Pharmacol. 2015;71:549–67.
Li M, Chen W, Sun X, Wang Z, Zou X, Wei H, et al. Metastatic colorectal cancer and severe hypocalcemia following irinotecan administration in a patient with X-linked agammaglobulinemia: a case report. BMC Medical Genetics. 2019;20(157):2–7.
Jai N, Patel JN, Papachristos A. Personalizing chemotherapy dosing using pharmacological methods. 2015;76:879–96.
Wilkinson DS. Therapeutic drug monitoring in oncology. Ther Drug Monit. 2019;41:551–2.
Hahn RZ, Antunes MV, Verza SG, Perassolo MS, Suyenaga ES, Schwartsmann G, et al. Pharmacokinetic and pharmacogenetic markers of irinotecan toxicity. Curr Med Chem. 2019;26:2085–107.
Meneghello A, Tartaggia S, Alvau MD, Polo F, Toffoli G. Biosensing technologies for therapeutic drug monitoring. Curr Med Chem. 2018;25:4354–77.
Rodríguez Cáceres MI, Durán-Merás I, Soto NEO, de Alba PLL, Martínez LL. Spectrofluorimetric determination of irinotecan in the presence of oxidant agents and metal ions. Talanta. 2008;74:1484–91.
Serrano LA, Yang Y, Salvati E, Stellacci F, Krol S, Guldin S. PH-mediated molecular differentiation for fluorimetric quantification of chemotherapeutic drugs in human plasma. Chem Commun. 2018;54:1485–8.
Tartaggia S, Alvau MD, Meneghello A, Casetta B, Polo F, Toffoli G. Practical fluorimetric assay for the detection of anticancer drug SN-38 in human plasma. J Pharm Biomed Anal. 2018;159:73–81.
De Bruijn P, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Determination of irinotecan (CPT-11) and its active metabolite SN-38 in human plasma by reversed-phase high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Appl. 1997;698:277–85.
Gravel E, Bourget P, Mercier L, Paci A. Fluorescence detection combined with either HPLC or HPTLC for pharmaceutical quality control in a hospital chemotherapy production unit: application to camptothecin derivatives. J Pharm Biomed Anal. 2005;39:581–6.
Poujol S, Pinguet F, Malosse F, Astre C, Ychou M, Culine S, et al. Sensitive HPLC-fluorescence method for irinotecan and four major metabolites in human plasma and saliva: application to pharmacokinetic studies. Clin Chem. 2003;49:1900–8.
Owens TS, Dodds H, Fricke K, Hanna SK, Crews KR. High-performance liquid chromatographic assay with fluorescence detection for the simultaneous measurement of carboxylate and lactone forms of irinotecan and three metabolites in human plasma. J Chromatogr B Anal Technol Biomed Life Sci. 2003;788:65–74.
Hahn RZ, Arnhold PC, Andriguetti NB, Schneider A, Klück HM, dos Reis SL, et al. Determination of irinotecan and its metabolite SN-38 in dried blood spots using high-performance liquid-chromatography with fluorescence detection. J Pharm Biomed Anal. 2018;150:51–8.
Bansal T, Awasthi A, Jaggi M, Khar RK, Talegaonkar S. Development and validation of reversed phase liquid chromatographic method utilizing ultraviolet detection for quantification of irinotecan (CPT-11) and its active metabolite, SN-38, in rat plasma and bile samples: application to pharmacokinetic studies. Talanta. 2008;76:1015–21.
Marangon E, Posocco B, Mazzega E, Toffoli G. Development and validation of a high-performance liquid chromatography-tandem mass spectrometry method for the simultaneous determination of Irinotecan and its main metabolites in human plasma and its application in a clinical pharmacokinetic study. PLoS One. 2015;10:1–18.
Gao S, Tao Z, Zhou J, Wang Z, Yun Y, Li M, et al. One-step solid extraction for simultaneous determination of eleven commonly used anticancer drugs and one active metabolite in human plasma by HPLC-MS/MS. Journal of Analytical Methods in Chemistry. 2018;7967694:1–12.
D'Aronco S, D'Angelo E, Crotti S, Traldi P, Agostini M. New mass spectrometric approaches for the quantitative evaluation of anticancer drugs levels in treated patients. 2019;41:1–10.
Saita T, Fujito H, Mori M. Development of ELISAs for irinotecan and its active metabolite SN-38. Biol Pharm Bull. 2000;23:911–6.
Kimmel DW, Leblanc G, Meschiewitz ME, Cliffel DE. Electrochemical sensors and biosensors. Anal Chem. 2012;84:685–707.
Karadas N, Sanli S, Akmese B, Dogan-Topal B, Can A, Ozkan SA. Analytical application of polymethylene blue-multiwalled carbon nanotubes modified glassy carbon electrode on anticancer drug irinotecan and determination of its ionization constant value. Talanta. 2013;115:911–9.
Zotti G, Berlin A, Vercelli B. Electrochemistry of conjugated planar anticancer molecules: irinotecan and sunitinib. Electrochim Acta. 2017;231:336–43.
Bonazza G, Tartaggia S, Toffoli G, Polo F, Daniele S. Voltammetric behaviour of the anticancer drug irinotecan and its metabolites in acetonitrile. Implications for electrochemical therapeutic drug monitoring. Electrochim Acta. 2018;289:483–93.
Novak JI, Komorsky-Lovrić Š, Lucić Vrdoljak A, Popović AR, Neuberg M. Voltammetric characterisation of anticancer drug irinotecan. Electroanalysis. 2018;30:336–44.
Temerk Y, Ibrahim M, Ibrahim H, Schuhmann W. Comparative studies on the interaction of anticancer drug irinotecan with dsDNA and ssDNA. RSC Adv. 2018;8:25387–95.
Norouzi P, Qomi M, Nemati A, Ganjali MR. Determination of anti colon cancer drug, irinotecan by fast Fourier transforms continuous cyclic voltammetry. Int J Electrochem Sci. 2009;4:1248–61.
Temerk YM, Ibrahim H. Individual and simultaneous square wave voltammetric determination of the anticancer drugs emodin and irinotecan at renewable pencil graphite electrodes. J Braz Chem Soc. 2013;10:1669–78.
Alvau MD, Tartaggia S, Meneghello A, Casetta B, Calia G, Serra PA, et al. Enzyme-based electrochemical biosensor for therapeutic drug monitoring of anticancer drug irinotecan. Anal Chem. 2018;90:6012–9.
Temerk YM, Ibrahim H, Schuhmann W. Square wave cathodic adsorptive stripping voltammetric determination of the anticancer drugs flutamide and irinotecan in biological fluids using renewable pencil graphite electrodes. Electroanalysis. 2016;28:372–9.
Hatamluyi B, Es’haghi Z, Modarres Zahed F, Darroudi M. A novel electrochemical sensor based on GQDs-PANI/ZnO-NCs modified glassy carbon electrode for simultaneous determination of Irinotecan and 5-fluorouracil in biological samples. Sensors Actuators B Chem. 2019;286:540–9.
Combes O, Barré J, Duché JC, Vernillet L, Archimbaud Y, Marietta MP, et al. In vitro binding and partitioning of irinotecan (CPT-11) and its metabolite, SN-38, in human blood. Investig New Drugs. 2000;18:1–5.
Kirstein MM, Lange A, Prenzler A, Manns MP, Kubicka S, Vogel A. Targeted therapies in metastatic colorectal cancer: a systematic review and assessment of currently available data. Oncologist. 2014;19:1156–68.
Braun MS, Seymour MT. Balancing the efficacy and toxicity of chemotherapy in colorectal cancer. Ther Adv Med Oncol. 2011;3:43–52.
Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37.
Cremolini C, Del Re M, Antoniotti C, Lonardi S, Bergamo F, Loupakis F, et al. DPYD and UGT1A1 genotyping to predict adverse events during first-line FOLFIRI or FOLFOXIRI plus bevacizumab in metastatic colorectal cancer. Oncotarget. 2018;9:7859–66.
Bard AJ, Faulkner LR. Electrochemical methods. Fundamental and applications. 2nd ed. New York: Wiley; 1980.
Long GL, Winefordner JD. Limit of detection: a closer look at the IUPAC definition. Anal Chem. 1983;55:713A–24A.
Pitot HC, Goldberg RM, Reid JM, Sloan JA, Skaff PA, Erlichman C, et al. Phase I dose-finding and pharmacokinetic trial of irinotecan hydrochloride (CPT-11) using a once-every-three-week dosing schedule for patients with advanced solid tumor malignancy. Clin Cancer Res. 2000;6:2236–44.
Xie R, Mathijssen RHJ, Sparreboom A, Verweij J, Karlsson MO. Clinical pharmacokinetics of irinotecan and its metabolites in relation with diarrhea. Clin Pharmacol Ther. 2002;72:265–75.
Kehrer DFS, Sparreboom A, Verweij J, De Bruijn P, Nierop CA, Van de Schraaf J, et al. Modulation of irinotecan-induced diarrhea by cotreatment with neomycin in cancer patients. Clin Cancer Res. 2001;7:1136–41.
Satoh T, Yasui H, Muro K, Komatsu Y, Sameshima S, Yamaguchi K, et al. Pharmacokinetic assessment of irinotecan, SN-38, and SN-38-glucuronide: a substudy of the FIRIS study. Anticancer Res. 2013;33:3845–54.
Sochor J, Dobes J, Krystofova O, Ruttkay-Nedecky B, Babula P, Pohanka M, et al. Electrochemistry as a tool for studying antioxidant properties. Int J Electrochem Sci. 2013;8:8464–89.
Chan KK, Webster RD. Solid phase extraction - voltammetric coupled detection of caffeine in acetonitrile. Electroanalysis. 2016;28:516–22.
Casella IG, Bonito R, Contursi M. Determination of some β-blockers by electrochemical detection on polycrstalline gold electrode after solid phase extraction (SPE). Electroanalysis. 2016;28:1060–7.
Xu S, Lin G, Zhao W, Wu Q, Luo J, Wei W, et al. Necklace-like molecularly imprinted nanohybrids based on polymeric nanoparticles decorated multiwalled carbon nanotubes for highly sensitive and selective melamine detection. ACS Appl Mater Interfaces. 2018;10:24850–9.
Amatatongchai M, Sroysee W, Sodkrathok P, Kesangam N, Chairam S, Jarujamrus P. Novel three-dimensional molecularly imprinted polymer-coated carbon nanotubes (3D-CNTs@MIP) for selective detection of profenofos in food. Anal Chim Acta. 2019;1076:64–72.
European Medicines Agency Committee for Medicinal Products for Human Use. Guideline on bioanalytical method validation. 2012;44:1–23 EMEA/CHMP/EWP/192217.
Funding
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) under the grant assigned for the Project 12214 (Innovative Tools for cancer risk assessment and early diagnosis—5 × 1000) and the Regione Friuli-Venezia-Giulia under the grant assigned for the Project “NADIATools” (Nano Diagnostic and Automated Therapeutic Tools for Oncology—POR-FESR 2014-2020, call 1.3b Smart Health).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For all experiments conducted with patient plasma, informed consent was obtained and approval was granted by the Medical Ethics Committee of the Centro di Riferimento Oncologico di Aviano (CRO), Italy.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 983 kb)
Rights and permissions
About this article
Cite this article
Bonazza, G., Tartaggia, S., Toffoli, G. et al. A fast method for the detection of irinotecan in plasma samples by combining solid phase extraction and differential pulse voltammetry. Anal Bioanal Chem 412, 1585–1595 (2020). https://doi.org/10.1007/s00216-020-02386-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02386-1