Abstract
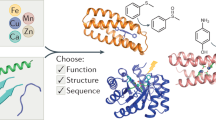
Ab initio in silico design of proteins and enzymes has emerged as a powerful tool to design application-tailored proteins and catalysts for a wide range of applications. Several enzymes exploit the unique features of metal cofactors to achieve catalytic activity otherwise unattainable through the use of only natural amino acid residues. One of the major bottlenecks in ab initio design of novel proteins relies on long-range and epistatic effects that severely limit the possibility of a rational design. Within this framework there is an ongoing effort to reduce protein length and complexity to unlock the full potential of in silico protein design. In this work we specifically address this problem designing and investigating the dynamic features of 10 in silico designed minimal metallo-proteins. In particular, in this paper we investigate whether and to what extent it is possible to design a minimal metallo-enzyme made of only residues involved in metal binding. In this research we address these questions by investigating the ability of 10 different “mini-proteins” with a length shorter than 15 residues. Molecular dynamics studies clearly show that it is possible to design a minimal protein able to bind a metal atom with the correct geometry. It is noteworthy that designed mini-proteins cannot achieve the formation of a canonical hydrophobic core, rather the metal ion provides a “metal core” around which the entire protein is organized. This opens the possibility of designing synthetic enzymes composed of only functional residues organized around a “metal core” which acts as both structural and functional determinat.







Similar content being viewed by others
References
Hardy LW, Malikayil A (2003) The impact of structure-guided drug design on clinical agents. Curr Drug Discov 3:15–20
Dwyer Mary A, Looger Loren L, Hellinga Homme W (2004) Computational design of a biologically active enzyme. Science 304:1967–1971
Röthlisberger D, Khersonsky O, Wollacott AM, Jiang L, DeChancie J, Betker J, Gallaher JL, Althoff EA, Zanghellini A, Dym O, Albeck S, Houk KN, Tawfik DS, Baker D (2008) Kemp elimination catalysts by computational enzyme design. Nature 453:190–195
Alexandrova AN, Roethlisberger D, Baker D, Jorgesen WL (2008) Catalytic mechanism and performance of computationally designed enzymes for kemp elimination. J Am Chem Soc 130:15907–15915
Faiella M, Andreozzi C, Martin T, de Rosales R, Pavone V, Maglio O, Nastri F, DeGrado WF, Lombardi A (2009) An artificial di-iron oxo-protein with phenol oxidase activity. Nat Chem Biol 5:882–884
Cozier GE, Leese MP, Lloyd MD, Baker MD, Thiyagarajan N, Acharya KR, Potter BV (2010) Structures of human carbonic anhydrase II/inhibitor complexes reveal a second binding site for steroidal and nonsteroidal inhibitors. Biochemistry 49:3464–3476
Tollin G, Hanson LK, Caffrey M, Meyer TE, Cusanovich MA (1986) Redox pathways in electron-transfer proteins: correlations between reactivities, solvent exposure, and unpaired-spin-density distributions. PNAS 83:3693–3697
Lee S, Doddapaneni K, Hogue A, McGhee L, Meyers S, Wu Z (2010) Solution structure of Gfi-1 zinc domain bound to consensus DNA. J Mol Biol 397:1055–1066
Tokuriki N, Stricher F, Serrano L, Tawfik DS (2008) How protein stability and new functions trade off. PLoS Comput Biol 4:e1000002
Bershtein S, Segal M, Bekerman R, Tokuriki N, Tawfik DS (2006) Robustness-epistasis link shapes the fitness landscape of a randomly drifting protein. Nature 444:929–932
Nagarajan H, Butler JE, Klimes A, Qiu Y, Zengler K, Ward J, Young ND, Methe BA, Palsson BO, Lovley DR, Barrett CL (2010) De Novo assembly of the complete genome of an enhanced electricity-producing variant of Geobacter sulfurreducens using only short reads. PLoS ONE 5:e10922
Remenant B, Coupat-Goutaland B, Guidot A, Cellier G, Wicker E, Allen C, Fegan M, Pruvost O, Elbaz M, Calteau A, Salvignol G, Mornico D, Mangenot S, Barbe V, Medigue C, Prior P (2010) Genomes of three tomato pathogens within the Ralstonia solanacearum species complex reveal significant evolutionary divergence. BMC Genomics 11:379
Rohl CA, Strauss CE, Misura KMS, Baker D (2004) Protein structure prediction using Rosetta. Meth Enzymol 383:66–93
Tainer JA, Getzoff ED, Beem KM, Richardson JS, Richardson DC (1982) Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol 160:181–217
James P, Braun C, Wang R, Gumbart W, Tajkhorshid J, Villa E, Chipot E, Skeel C, Kalé DR, Schulten L, Klaus S (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802
MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B 102:3586–3616
Jorgensen WL, Chandrasekhar J, Madura J, Impley RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Ungar L, Scherer N, Voth G (1997) Classical molecular dynamics simulation of the photoinduced electron transfer dynamics of plastocyanin. Biophys J 72:5–17
Ullmann GM, Knapp EW, Kostić NM (1997) Computational simulation and analysis of dynamic association between Plastocyanin and Cytochrome f. Consequences for the Electron-Transfer Reaction. J Am Chem Soc 119:42–52
Wiesemann F, Teipel S, Krebs B, Howeler U (1994) Force field calculations on the structures of transition metal complexes. Application to Copper(II) Complexes in Square-Planar Coordination. Inorg Chem 33:1891–1898
Nordlund A, Leinartaite L, Sarabojia K, Aisenbreyc C, Gro G, Zetterstro P, Danielssona J, Loganb DT, Oliveberga M (2009) Functional features cause misfolding of the ALS-provoking enzyme SOD1. PNAS 106:9667–9672
Sakharov DV, Carmay L (2005) Zn protein simulations including charge transfer and local polarization effects. J Am Chem Soc 127:4921–4929
Batinić-Haberle I, Rebouças JS, Spasojević I (2010) Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal 13:877–918
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
The picture shows the three-dimensional structure of protein 002. In orange the ion copper. Figure created with Chimera software (20) (DOC 163 kb)
Table S1
Relative distance between Glu 6 and His 8 of two independent simulations of protein 010 with copper ion. Glu6_a and Glu6_b indicate, respectively, the two tritratable position of the glutamate residue. Distances are in angstrom (DOC 41 kb)
Rights and permissions
About this article
Cite this article
Mazzucco, N., Zanconato, S., De Lucrezia, D. et al. Design and dynamic simulation of minimal metallo-proteins. J Mol Model 17, 2919–2925 (2011). https://doi.org/10.1007/s00894-011-0993-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-011-0993-8