Abstract
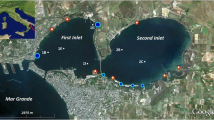
Parameters of ecosystem structure and functioning were analyzed in three hypereutrophic lagoons of Ca’Pisani during the season of 2001. Lagoons are situated at wetlands of the NW Adriatic in the vicinity of the Porto Viro, Po River delta. They are associated with intensive fish culture enterprise and accept its wastewater. In June, the lagoons were found overloaded with the biomass of nitrophylic algae. At the end of July, a bloom of potentially toxic dinoflagellate Alexandrium tamarense occurred. Soon, it was supplanted by the picocyanobacterial assemblage, which arrived into the lagoons from the coastal Adriatic via the Marine channel. Wet biomass of this new picocyanobacterial bloom arrived in September attained 30–60 g m−3. Decrease of white disk water transparency down to 30–40 cm resulted in a gross mortality of macrophytes accompanied by spreading of floating saprobic alga Enteromorpha. Phytoplankton was dominated in June to July by small mixotrophic phytoflagellates with a wet biomass of 200–1300 mg m−3. Number of bacterioplankton ranged between 4 and 7 × 106 ml−1 and its wet biomass between 1.4 and 2.1 g m−1. Its maximum of 18 × 106 ml−1 was observed in late August, when the mortality of macrophytes had occurred. Zooplankton and zoobenthos were found depleted in the lagoons especially during the blooms. Diel fluctuations of dissolved oxygen in the lagoons in June to July reached 150–200% of saturation. Photosynthetic oxygen production ranged between 15 and 30 g O2 m−2 d−1. Water column deoxygenation rate was 1–1.5 mg O2 l−1 h−1. Total photosynthesis production reached 3–8 g C m−2 d−1 by the share of phytoplankton 5–15%. Hyper-accumulation of total phosphorus in the water column and of toxic labile sulfides in the bottom sediments was documented. Content of inorganic phosphorus in water remained unusually high even by its intensive uptake by microplankton. The PO4P uptake rate measured with 32P-label ranged during the bloom of picocyanobacteria between 10 and 50 nM l−1 min−1, and the residence time of PO4P between 15 and 50 min. The data were generalized via the calculation of energy balance and the deduction of the energy flow scheme in the ecosystem. Their analysis demonstrates the invalidation of ecosystems in hypereutrophic lagoons due to their overload with organic matter, with nutrients and with labile sulfides. After having depleted their animal food web, they are unable to decompose local plus external organic loading.
Similar content being viewed by others
References
Al-Layl K. M. (2002). Phylogenetic analysis of toxic cyanobacterium Synechocystis sp isolated from Makkah Saud, Saudi Arabia. Journal of Science Medicine and Engineering 14(1): 23–30
Carmouse J. D., Elia D. S., Silva S. and Azevedo L. S. (1989). Ecological changes in Brazilian lagoon related to the dystrophic crisis. Verhandliungen International Ver Limnologie 251: 21–35
Caron D. A. (1983). Technique for enumeration of nanoplankton using epifluorescence microscopy. Limnology and Oceanography 33: 1595–1606
Carreto J. R., Benavides H. R., Negri R. M. and Glorioso P. D. (1986). Toxic red tide in the Argentina Sea. Journal of Plankton Research 8: 15–28
Chua T. E., Pavi J. M. and Guahn F. Y. (1989). Environmental impact of aquaculture, and effect of pollution effluents on coastal aquaculture. Marine Pollution Bulletin 20(7): 335–343
Ghion F. and Guidasti R. (1984). La vallicoltura integrata. In: Alessandra, G. (eds) Richerca Sperimentazione in Aquacoltura, pp 19–58. E.S.A.V, Venezia
Goldberg E. D. (1995). Problems in the coastal zone for 21 Century. Marine Pollution Bulletin 31: 132–138
Gray J. S. and Wurs G. R. (2002). Effect of hypoxia and organic enrichment on the coastal marine environment. Marine Ecology Progress Series 238: 249–279
Hobbie J. E., Daley R. J. and Jasper S. (1977). Use of Nuclepore filters for counting bacteria by epifluorescence microscopy. Applied and Environmental Microbiology 33: 1225–1228
Meyer-Reil L. A. and Koster M. (2000). Eutrophication of marine waters: effects on benthic communities. Marine Pollution Bulletin 41: 255–263
Mitsui A. D. and Goodman A. (1989). Haemolythic toxins in marine cyanobacterium Synechococcus sp. In: Okaichi, T. (eds) Red Tides. Proceedings 1st International Symposium, pp 367–370. Elsevier, New York
Nixon S. W. (1992). Coastal marine eutrophication. Ophelia 41: 199–219
Oudra B., Loudiki M. and Vackon V. (2002). Detection and quantification of microcystins from cyanobacteria isolated from reservoirs in Morocco. Environmental Technology 17: 32–39
Parsons T. R., Maita Y. and Lalli C. U. (1984). A Manual of Chemical and Biological Methods for Sea water Analysis. Pergamon, New York, 420
Pearson T. H. and Rosenberg R. (1978). Macrobenthic succession in relation to organic enrichment and pollution. Ocenography and Marine Biology Annual Review 16: 229–311
Peckol P. and Rivers J. S. (1995). Responses of macroalgae cladophora and gracilaria to environmental stress and eutrophication. Journal of Experimental Marine Biology and Ecology 190: 1–16
Ravagnan G. (1978). Vallicoltura Marina. Erdagricole, Milano, 238
Ravagnan G. (1991). Vallicoltura Moderna. Erdagricole, Milano, 350
Riegman R. and Mur L. R. (1986). Phytoplankton growth and phosphate uptake for P-limitation. Limnology and Oceanography 31: 983–988
Romankevich E. A. (1977). Geochemistry of Organic matter in the Ocean. Nauka, Moscow, 450
Rosenberg C. M. (1985). Eutrophication – the future marine coastal nuisance. Marine Pollution Bulletin 16: 227–231
Sorokin Yu. I. (1999). Radioisotopic Methods in Hydrobiology. Springer, Heidelberg, 326
Sorokin Yu. I. and Boscolo R. (2002). La moria di vongole nell’ estate 2001 in Laguna di Venezia. Revista di Studi e Researche (Chiggia) 20: 55–60
Sorokin Yu. I., Sorokin Yu. P., Giovanardi O. and Dallavenezia L (1996a). Study the Venice lagoon ecosystem with the emphasis on anthropogenic impact. Marine Ecology Progress Series 141: 247–261
Sorokin Yu. I., Sorokin Yu. P. and Gnes A. (1996b). Structure and functioning of anthropogenically transformed Comacchio lagoon ecosystem. Marine Ecology Progress Series 133: 57–71
Sorokin Yu. I., Dallocchio F., Gelli F. and Pregnolato L. (1996c). Phosphorus metabolism in anthropogenically transformed lagoon ecosystems: Comacchio lagoons. Journal of Sea Research 35: 243–252
Sorokin Yu. I., Sorokin Yu. P. and Ravagnan G. (1999). Analysis of lagoon ecosystems in the Po River delta, associated with the intensive aquaculture. Estuarine Coastal and Shelf Science 48: 325–334
Sorokin Yu. I., Sorokin P. Yu. and Ravagnan G. P. (2002). On the changing ecology of Venice lagoon. Hydrobiologia 487: 1–18
Sorokin P. Yu., Sorokin Yu. I., Boscolo R. and Giovanardi O (2004a). Bloom of picocyanobacteria in the Venice lagoon during summer - autumn 2001: ecological sequences. Hydrobiologia 523: 71–85
Sorokin Yu. I., Sorokin Yu. P., Zakuskina O. Yu. and Dallocchio F. (2004b). Features of hypereutrophic Molino lagoon dominated by sedentary polychaetes. Hydrobiologia 521: 189–200
Viaroli P. R., Azzoni R., Bartoli M., Giordani G. and Taje L. (2001). Dystrophic outbrakes in the Sacca di Goro lagoon. In: Farranda, E. M. (eds) Mediterranean Ecosystems: Structures and Processes, pp 340–355. Springer, Italia
Vollenveider R. A., Rinaldi A. and Montanari G. (1992). Eutrophication: results of a ten years monitoring along the Emilia Romagna coast. In: Vollenveider, R., Marchetti, R., and Viviani, R. (eds) Marine Coastal Eutrophication, pp 63–06. Elseivier, Amsterdam
Zevenboom W. and Murm L. R (1982). Assessment of factors limiting growth rate of Oscillatoria agardhii in hyperelitrophic lake Wolderwide. Limnology and Oceanography 27: 39–52
Zsolnay J. (1975). Total organic carbon in the Baltic Sea as estimated by BOD. Marine Biology 29: 125–128
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sorokin, Y.I., Sorokin, P.Y. & Ravagnan, G. Hypereutrophication events in the Ca’Pisani lagoons associated with intensive aquaculture. Hydrobiologia 571, 1–15 (2006). https://doi.org/10.1007/s10750-006-0250-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-006-0250-9