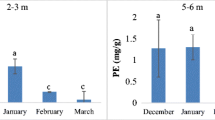
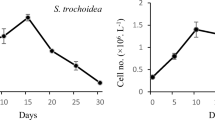
Abstract
Five red algae, Agardhiella subulata, Gracilariopsis longissima, Gracilaria vermiculophylla, Polysiphonia morrowii, and Pyropia elongata were sampled in winter for the extraction of phycoerythrin. The extracted phycoerythrin molecules were compared with the commercial phycoerythrin to determine the intrinsic fluorescence characteristics of the single pigments. An improved selective method for phycoerythrin extraction was set up for routinary investigation. The use of the mortar and pestle method for tissue homogenization with a freeze-thawing cycle allowed a simple and complete homogenization of the red algae. The extraction of phycoerythrin with diluted EDTA solutions (1 mM) at pH 9 enabled a selective and easy extraction of the pigment with 95–98% extraction efficiency. The way pH affected the phycoerythrin, phycocyanin, and allophycocyanin selective extraction was also evaluated. The 3D fingerprint of each pigment was recorded, and a comparison of different phycoerythrin spectra was performed by fluorescence spectroscopy highlighting differences in A. subulata and P. morrowii phycoerythrins in comparison with commercial standards purified from Pyropia. The productivity and the advantages of phycoerythrin that was extracted from unattached red algal species are discussed.





Similar content being viewed by others
References
Algarra P, Thomas JC, Mousseau A (1990) Phycobilisome heterogeneity in the red alga Porphyra umbilicalis. Plant Physiol 92:570–576
Beer S, Eshel A (1985) Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aust J Mar Freshw Res 36:785–792
Bermejo R, Acién FG, Ibanez MJ, Fernandez JM, Molina E, Alvarez-Pez JM (2003) Preparative purification of B-phycoerythrin from the microalga Porphyridium cruentum by expanded-bed adsorption chromatography. J Chromatogr B 790:317–325
Benavides J, Palomares MR (2006) Simplified two-stage method to B-phycoerythrin recovery from Porphyridium cruentum. J Chromatogr B 844:39–44
Bryant DA (1982) Phycoerythrocyanin and phycoerythrin: properties and occurrence in cyanobacteria. Microbiology 128:835–844
Cai C, Wang Y, Li C, Guo Z, Jia R, WU W, Hu Y, He P (2014) Purification and photodynamic bioactivity of phycoerythrin and phycocyanin from Porphyra yezoensis Ueda. J Ocean Univ China 12:479–484
Chang WR, Jiang T, Wan ZL, Zhang JP, Yang ZX, Liang DC (1996) Crystal structure of R-phycoerythrin from Polysiphonia urceolata at 2.8 Å resolution. J Mol Biol 262:721–731
Dumay J, Clément N, Morançais M, Fleurence J (2013) Optimization of hydrolysis conditions of Palmaria palmata to enhance R-phycoerythrin extraction. Bioresour Technol 131:21–27
Edding M, Macchiavello J, Black H (1987) Culture of Gracilaria sp. in outdoor tanks: productivity. Hydrobiologia 151/152:369–373
European Patent Office (2017) Espacenet patent search engine. https://worldwide.espacenet.com; searched on 17 May 2017
Francavilla M, Franchi M, Monteleone M, Caroppo C (2013) The red seaweed Gracilaria gracilis as a multi products source. Mar Drugs 11:3754–3776
Glazer AN, Fang S (1973) Chromophore content of blue-green algal phycobiliproteins. J Biol Chem 248:659–662
Glazer AN, Hixson CS (1975) Characterization of R-phycocyanin. Chromophore content of R-phycocyanin and C-phycoerythrin. J Biol Chem 250:5487–5495
González-Ramírez E, Andújar-Sánchez M, Ortiz-Salmerón E, Bacarizo J, Cuadri C, Mazzuca-Sobczuk T, Ibáñez MJ, Cámara-Artigas A, Martínez-Rodríguez S (2014) Thermal and pH stability of the B-phycoerythrin from the red algae Porphyridium cruentum. Food Biophys 9:184–192
Huang YM, Rorrer GL (2002) Dynamics of oxygen evolution and biomass production during cultivation of Agardhiella subulata microplantlets in a bubble-column photobioreactor under medium perfusion. Biotechnol Prog 18:62–71
Hugh DJM (2003) A guide to seaweed industry. FAO Fisheries Technical Paper 441, Rome
Ismail MM, Osman MEH (2016) Seasonal fluctuation of photosynthetic pigments of most common red seaweeds species collected from Abu Qir, Alexandria, Egypt. Rev Biol Mar Oceanogr 51:515–525
Kao O, Berns DS, Maccoll R (1971) C-Phycocyanin monomer molecular weight. Eur J Biochem 19:595–599
Kawsar S, Fujii Y, Matsumoto R, Yasumitsu H, Ozeki Y (2011) Protein R-phycoerythrin from marine red alga Amphiroa anceps: extraction, purification and characterization. Phytol Balcan 17(3):347–354
Lauceri R, Bresciani M, Lami A, Morabito G (2017) Chlorophyll a interference in phycocyanin and allophycocyanin spectrophotometric quantification. J Limnol. https://doi.org/10.4081/jlimnol.2017.1691
Ley AC, Butler WL (1977) Isolation and function of allophycocyanin B of Porphyridium cruentum. Plant Physiol 59:974–980
Liu LN, Chen XL, Zhang XY, Zhang YZ, Zhou BC (2005) One-step chromatography method for efficient separation and purification of R-phycoerythrin from Polysiphonia urceolata. J Biotechnol 116:91–100
Liu LN, Su HN, Yan SG, Shao SM, Xie BB, Chen XL, Zhang XY, Zhou BC, Zhang YZ (2009) Probing the pH sensitivity of R-phycoerythrin: investigations of active conformational and functional variation. Biochim Biophys Acta Bioenerg 1787:939–946
Mclachlan J, Bird CJ (1986) Gracilaria (Gigartinales, Rhodophyta) and productivity. Aquat Bot 26:27–49
Menges F (2016) Spekwin32—optical spectroscopy software, Version 1.72.0. http://www.effemm2.de/spekwin/; searched on 11 August 2016
Mensi F, Ksouri J, Seale E, Romdhane MS, Fleurence J (2012) A statistical approach for optimization of R-phycoerythrin extraction from the red algae Gracilaria verrucosa by enzymatic hydrolysis using central composite design and desirability function. J Appl Phycol 24:915–926
Moraes CC, Kalil SJ (2009) Strategy for a protein purification design using C-phycocyanin extract. Bioresour Technol 100:5312–5317
Moreth CM, Yentsch CS (1970) A sensitive method for the determination of open ocean phytoplankton phycoerythrin pigments by fluorescence. Limnol Oceanogr 15:313–317
Munier M, Jubeau S, Wijaya A, Morançais M, Dumay J, Marchal L, Jaouen P, Fleurence J (2014) Physicochemical factors affecting the stability of two pigments: R-phycoerythrin of Grateloupia turuturu and B-phycoerythrin of Porphyridium cruentum. J Food Chem 150:400–407
Nguyen HPT, Morançais M, Fleurence J, Dumay J (2017) Mastocarpus stellatus as a source of R-phycoerythrin: optimization of enzyme assisted extraction using response surface methodology. J Appl Phycol 29:1563–1570
Niu JF, Wang GC, Tseng CK (2006) Method for large-scale isolation and purification of R-phycoerythrin from red alga Polysiphonia urceolata Grev. Protein Expr Purif 46:23–31
Oi VT, Glazer AN, Stryer L (1982) Fluorescent phycobiliprotein conjugates for analysis of cells and molecules. J Cell Biol 93:981–986
Ogawa H, Haruo M, Takahide S, Yoshihiro Y, Tuyosi O, Naomichi I (1991) Effects of pH on the conformation of phycoerythrin from nori Porphyra sp. Nippon Suisan Gakk 57:899–903
Pan Q, Chen M, Li J, Wu Y, Zhen C, Liang B (2013) Antitumor function and mechanism of phycoerythrin from Porphyra haitanensis. Biol Res 46:87–95
Ramirez AO, Merrill JE, Smith DM (2000) pH affects the thermal inactivation parameters of R-phycoerythrin from Porphyra yezoensis. J Food Sci 65:1046–1050
Rowan KS (1989) Photosynthetic pigments of algae. Cambridge University Press, Melbourne
Sasim SM, Egiert JS, Kosakowska A (2014) Quantitative analysis of extracted phycobilin pigments in cyanobacteria—an assessment of spectrophotometric and spectrofluorometric methods. J Appl Phycol 26:2065–2074
Sfriso AA, Gallo M, Baldi F (2016a) Carbohydrate and agar yield: preliminary insights on seasonal variations in Ulva and three Gracilariaceae. Biol Mar Mediterr 23:162–166
Sfriso AA, Gallo M, Baldi F (2017a) Seasonal variation and yield of sulfated polysaccharides in seaweeds from the Venice Lagoon. Bot Mar 60:339–349
Sfriso AA, Sfriso A (2017) In situ biomass production of Gracilariaceae and Ulva rigida: the Venice Lagoon as a study case. Bot Mar 60:271–283
Sfriso A, Buosi A, Facca C, Sfriso AA (2017b) Role of environmental factors in affecting macrophyte dominance in transitional environments: the Italian Lagoons as a study case. Mar Ecol 38(2):e12414. https://doi.org/10.1111/maec.12414
Sfriso A, Facca C, Bon D, Buosi A (2016b) Macrophytes and ecological status assessment in the Po delta transitional systems, Adriatic Sea (Italy). Application of Macrophyte Quality Index (MaQI). Acta Adriat 57(2):209–226
Sfriso A, Marcomini A, Pavoni B (1994a) Gracilaria distribution, production and composition in the lagoon of Venice. Bioresour Technol 50:165–173
Sfriso A, Marcomini A, Pavoni B (1994b) Distribution, production and composition of Gracilaria in the central lagoon of Venice. COST-48 Symposium of Sub Group III, Trieste, pp 1–17
Soltzberg LJ, Lor S, Okey-Igwe N, Newman R (2012) 3D fluorescence characterization of synthetic organic dyes. Am J Anal Chem 3:622–631
Sonania RR, Singhb NK, Kumarc J, Thakara D, Madamwara D (2014) Concurrent purification and antioxidant activity of phycobiliproteins from Lyngbya sp. A09DM: an antioxidant and anti-aging potential of phycoerythrin in Caenorhabditis elegans. Process Biochem 49:1757–1766
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Toffanin R, Cecere E, Rizzo R, Knutsen SH (1997) Investigation of the carrageenans extracted from Solieria filiformis and Agardhiella subulata from Mar Piccolo, Taranto. Mar Chem 58:319–325
Vilar VJP, Botelho CMS, Boaventura RAR (2006) Equilibrium and kinetic modelling of Cd(II) biosorption by algae Gelidium and agar extraction algal waste. Water Res 40:291–302
Vilar VJP, Botelho CMS, Boaventura RAR (2005) Influence of pH, ionic strength and temperature on lead biosorption by Gelidium and agar extraction algal waste. Process Biochem 40:3267–3275
Viskari PJ, Colyer CL (2003) Rapid extraction of phycobiliproteins from cultured cyanobacteria samples. Anal Biochem 319:263–271
Zimba PV (2012) An improved phycobilin extraction method. Harmful Algae 17:35–39
Zhongzheng P, Baicheng Z, Chengkui Z, Tseng CK (1987) The effect of pH on both spectral types of R-phycoerythrin. Chin J Oceanol Limnol 5(1):73–79
Wang L, Qu Y, Fu X, Zhao M, Wang S, Sun L (2014) Isolation, purification and properties of an R-phycocyanin from the phycobilisomes of a marine red macroalga Polysiphonia urceolata. PLoS One 9(2):e87833
Wang L, Wang S, Fu X, Sun L (2015) Characteristics of an R-Phycoerythrin with two γ subunits prepared from red macroalga Polysiphonia urceolata. PLoS One 10(3):e0120333
Wiley PS, Neefus CD (2007) An improved method for estimating R-phycoerythrin and R-phycocyanin contents from crude aqueous extracts of Porphyra (Bangiales, Rhodophyta). J Appl Phycol 19:123–129
Acknowledgments
The authors are grateful to Dr. Orietta Zucchetta for the English editing and to Prof. Adriano Sfriso for his taxonomical expertise and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sfriso, A.A., Gallo, M. & Baldi, F. Phycoerythrin productivity and diversity from five red macroalgae. J Appl Phycol 30, 2523–2531 (2018). https://doi.org/10.1007/s10811-018-1440-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1440-3