Abstract
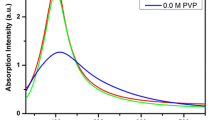
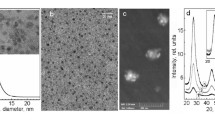
We have prepared, for the first time, stable and uncapped Ag/Cu-based bifunctional nanoparticles (NPs) (BFNPs) in water, by combining ps laser ablation in liquid environment and galvanic replacement. The particles were obtained in a single step by 1,064 nm irradiation of a Cu target in water solutions of AgNO3 or AgNO2. Under proper salt concentration and irradiation conditions, the laser beam activates formation of deep orange colloids, which are positively charged and stable for weeks. High resolution transmission electron microscopy (HRTEM) analysis showed a predominance of composite crystalline nanostructures with size in the 1–15 nm range and consisting of fcc Ag and fcc Cu (or its oxides). While CuO tenorite crystalline phase was detected by HRTEM, X-ray photoelectron spectroscopy analysis permitted to observe also the Cu(I) oxidation state of Cu, being the Cu(I)/Cu(II) ratio different in the samples obtained in AgNO3 or AgNO2 baths. Functionalization with organic ligands and subsequent Raman tests demonstrated the SERS activity of the BFNPs and the existence of different complexing surface sites.









Similar content being viewed by others
References
Alloisio M, Demartini A, Cuniberti C, Dellepiane G, Jadhav S, Thea S, Giorgetti E, Gellini C, Muniz-Miranda M (2009) Polydiacetylene-functionalized noble metal nanocages. J Phys Chem C 113:19475–19481. doi:10.1021/jp905787h
Alvarez-Paneque AF, Rodrìguez-Gonzàlez B, Pastoriza-Santos I, Liz-Marzàn LM (2012) Shape-templated growth of Au@Cu nanoparticles. J Phys Chem C. doi:10.1021/jp3062724
Biesinger MC, Lau LWM, Gerson AR, Smart RStC (2010) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl Surf Sci 257:887–898. doi:10.1016/j.apsusc.2010.07.086
Boyer P, Ménard D, Meunier M (2010) Nanoclustered Co–Au particles fabricated by femtosecond laser fragmentation in liquids. J Phys Chem C 115:13497–13500. doi:10.1021/jp912162g
Bracey CL, Ellis PR, Hutchings CJ (2009) Application of copper–gold alloys in catalysis: current status and future perspectives. Chem Soc Rev 38:2231–2243. doi:10.1039/b817729p
Canton P, Pietropolli Charmet A (2010) Lattice measurements in metallic nanoparticles by means of HRTEM images. In: XXIV national conference of the Italian Chemical Society. ISBN 9788883050855
Caporali S, Chiappe C, Ghilardi T, Pomelli CS, Pinzino C (2012) Coordination environment of highly concentrated solutions of Cu(II) in ionic liquids through a multidisciplinary approach. Chem Phys Chem 13:1885–1892. doi:10.1002/cphc.201100876
Chatterjee K, Das D, Chakravorty D (2005) Optical absorption in composites containing copper core–copper-oxide shell nanostructure in a silica gel. J Phys D 38:451–455. doi:10.1088/0022-3727/38/3/015
Chen YH, Tseng YH, Yeh CS (2002) Laser-induced alloying Au–Pd and Ag–Pd colloidal mixtures: the formation of dispersed Au/Pd and Ag/Pd nanoparticles. J Mater Chem 12:1419–1422. doi:10.1039/B200587E
deRuijter WJ (1994) Measurement of lattice-fringe vectors from digital HREM images: theory and simulations. J Comput Assist Microsc 6:195–212
Giammanco F, Giorgetti E, Marsili P, Giusti A (2010) Experimental and theoretical analysis of photofragmentation of Au nanoparticles by picosecond laser radiation. J Phys Chem C 114:3354–3363. doi:10.1021/jp908964t
Giorgetti E, Giammanco F, Marsili P, Giusti A (2011) Effect of picosecond postirradiation on colloidal suspensions of differently capped AuNPs. J Phys Chem C 115:5011–5020. doi:10.1021/jp108042m
Giorgetti E, Muniz-Miranda M, Marsili P, Scarpellini D, Giammanco F (2012a) Stable gold nanoparticles obtained in pure acetone by laser ablation with different wavelengths. J Nanopart Res 14:648. doi:10.1007/s11051-011-0648-9
Giorgetti E, Marsili P, Muniz-Miranda M, Gellini C, Canton P, Giammanco F (2012b) Synthesis of colloidal nanoparticles of metals or metal/oxides by pulsed laser ablation in liquids. In: Materials today virtual conference: nanotechnology, 11–13 Dec 2012
Gonzalo J, Babonneau D, Afonso CN, Barnes JP (2004) Optical response of mixed Ag–Cu nanocrystals produced by pulsed laser deposition. J Appl Phys 96:5163–5168. doi:10.1063/1.1792810
Hahn A, Günther S, Wagener P, Barcikowski S (2011) Electrochemistry-controlled metal ion release from silicone elastomer nanocomposites through combination of different metal nanoparticles. J Mater Chem 21:10287–10289. doi:10.1039/c0jm04480f
Hirai M, Kumar A (2006) Wavelength tuning of surface plasmon resonance by annealing silver–copper nanoparticles. J Appl Phys 100:14309. doi:10.1063/1.2214213
Jo YK, Wen SB (2011) Direct generation of core/shell nanoparticles from double-pulse laser ablation in a background gas. J Phys D 44:305301. doi:10.1088/0022-3727/44/30/305301
Kazakevich PV, Simakin AV, Voronov VV, Shafeev GA (2006) Laser induced synthesis of nanoparticles in liquids. Appl Surf Sci 252:4373–4380. doi:10.1016/j.apsusc.2005.06.059
Kim K, Kim KL, Shin KS (2011a) Coreduced Ag/Pd bimetallic nanoparticles: surface enrichment of Pd revealed by Raman spectroscopy. J Phys Chem C 115:14844–14851. doi:10.1021/jp203160z
Kim K, Kim KL, Shin KS (2011b) Coreduced Pt/Ag alloy nanoparticles: surface enhanced Raman scattering and electrocatalytic activity. J Phys Chem C 115:23374–23380. doi:10.1021/jp2063707
Kvitek L, Panacek A, Soukupova J, Kolar M, Vecerova R, Prucek R (2008) Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J Phys Chem C 112:5825–5834. doi:10.1021/jp711616v
Liang EJ, Kiefer W (1996) Chemical effect of SERS with near-infrared excitation. J Raman Spectrosc 27:879–885
Liang EJ, Engert C, Kiefer W (1993) Surface-enhanced Raman scattering of pyridine in silver colloids excited in the near-infrared region. J Raman Spectrosc 24:775. doi:10.1002/jrs.1250241109
Liang EJ, Ye XL, Kiefer W (1997) Surface-enhanced Raman spectroscopy of crystal violet in the presence of halide and halate ions with near-infrared wavelength excitation. J Phys Chem A 101:7330–7335
Lin F, Yang J, Lu SH, Niu KY, Liu Y, Sun J, Du XW (2010) Laser synthesis of gold/oxide nanocomposites. J Mater Chem 20:1103–1106. doi:10.1039/b918945a
Liu R, Sen A (2012) Unified synthetic approach to silver nanostructures by galvanic displacement reaction on copper: from nanobelts to nanoshells. Chem Mater 24:48–54. doi:10.1021/cm2017714
Lu X, Chen J, Skrabalak SE, Xia Y (2008) Galvanic replacement reaction: a simple and powerful route to hollow and porous metal nanostructures. Proc Inst Mech Eng N J Nanoeng Nanosyst 221:1–16. doi:10.1243/17403499JNN111
Moretti G (1998) Auger parameter and Wagner plot in the characterization of chemical states by X-ray photoelectron spectroscopy: a review. J Electron Spectrosc Relat Phenom 95:95–144
Moskovits M, Vlčková B (2005) Adsorbate-induced silver nanoparticle aggregation kinetics. J Phys Chem B 109:14755–14758. doi:10.1021/jp051177o
Moulder JF, Stickle WF, Sobol PE, Bomben KD (1992) In: Chastain J (ed) Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corporation, Eden Prairie
Muniz-Miranda M (2000) Surface enhanced Raman scattering and normal coordinate analysis of 1,10-phenanthroline adsorbed on silver sols. J Phys Chem A 104:7803–7810
Muniz-Miranda M, Gellini C, Giorgetti E (2011) Surface-enhanced Raman scattering from copper nanoparticles obtained by laser ablation. J Phys Chem C 115:5021–5027. doi:10.1021/jp1086027
Muniz-Miranda M, Gellini C, Simonelli A, Tiberi M, Giammanco F, Giorgetti E (2012) Characterization of copper nanoparticles obtained by laser ablation in liquids. Appl Phys A. doi:10.1007/s00339-012-7160-7
Pandey P, Merwyn S, Agarwal GS, Tripathi BK, Pant SC (2012) Electrochemical synthesis of multi-armed CuO nanoparticles and their remarkable bactericidal potential against waterborne bacteria. J Nanopart Res 14:709. doi:10.1007/s11051-011-0709-0
Panigrahi S, Kundu S, Kumar Gosh S, Nath S, Praharaji S, Basu S, Pal T (2006) Selective one-pot synthesis of copper nanorods under surfactantless condition. Polyhedron 25:1263–1269. doi:10.1016/j.poly.2005.09.006
Qiu C, Zhang L, Wang H, Hiang C (2012) Surface-enhanced Raman scattering on hierarchical porous cuprous oxide nanostructures in nanoshell and thin-film geometries. Phys Chem Lett 3:651–657. doi:10.1021/jz201694s
Rice KP, Walker EJ, Stoykovich MP, Saunders AE (2011) Solvent-dependent surface plasmon response and oxidation of copper nanocrystals. J Phys Chem C 115:1793–1799. doi:10.1021/jp110483z
Tilaki RM, Irajizad A, Mahdavi SM (2007) Size, composition and optical properties of copper nanoparticles prepared by laser ablation in liquids. Appl Phys A 88:415–419. doi:10.1007/s00339-007-4000-2
Toshima N, Yonezawa T (1998) Bimetallic nanoparticles—novel materials for chemical and physical applications. New J Chem 22:1179–1201. ISSN: 11440546
Walther A, Müller A (2008) Janus particles. Soft Matter 4:663–668. doi:10.1039/b718131k
Acknowledgments
Funds from the Project NABLA (Decree n.4508-September 1, 2010 by Regione Toscana-Italy, PAR FAS 2007–2013 funds, Action 1.1.a.3) and Project PRIN2009 “Novel plasmon-based processes and materials for sensor applications” of the Italian Ministry of Research are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Giorgetti, E., Marsili, P., Canton, P. et al. Cu/Ag-based bifunctional nanoparticles obtained by one-pot laser-assisted galvanic replacement. J Nanopart Res 15, 1360 (2013). https://doi.org/10.1007/s11051-012-1360-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1360-0