Abstract
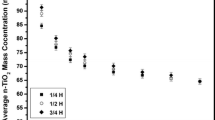
Long-term stability of two engineered nanomaterials (ENMs), i.e., the inorganic n-TiO2 and the organic Multi-Walled Carbon Nanotubes (MWCNTs), dispersed in artificial freshwater (5–100 mg l−1), was investigated from short-term settling velocity, particle size distribution, and surface charge. Hydrodynamic diameter and ζ-pot, calculated by means of dynamic and electrophoretic light scattering, respectively, qualitatively indicated a general ENMs dispersion instability over 1 h time. Sedimentation results, obtained by centrifugal separation analysis using the LUMiSizer over approx. 30 min analysis time, allowed to estimate the quantitative long-term (over 30 days) stability of ENMs. Settling data fitted satisfactorily with a first-order kinetic equation (R 2 in the range of 0.918–0.989). The settling rate constant k values extrapolated at gravity spanned one order of magnitude, i.e., from 7.21 × 10−5 to 4.12 × 10−4 s−1, and with the increasing of initial ENMs concentration. Sedimentation velocities were in good agreement with short- to long-term literature data (7.8 × 10−2–1.7 × 10−1 m day−1 vs. 5 × 10−4–3 × 10−1 m day−1 for n-TiO2 and 5.9 × 10−2–3.4 × 10−1 m day−1 vs. 2 × 10−1–1.2 m day−1 for MWCNTs). n-TiO2 showed a higher long-term stability with respect to MWCNTs (average: 1 × 10−1 ± 3.4 × 10−2 m day−1 instead of 1.7 × 10−1 ± 1.1 × 10−1 m day−1, respectively).






Similar content being viewed by others
References
Adeleye AS, Keller AA (2014) Long-term colloidal stability and metal leaching of single wall carbon nanotubes: effect of temperature and extracellular polymeric substances. Water Res 49:236–250. doi:10.1016/j.watres.2013.11.032
Afrooz ARMN, Khan IA, Hussain SM, Saleh NB (2013) Mechanistic heteroaggregation of gold nanoparticles in a wide range of solution chemistry. Environ Sci Technol 47:1853–1860
Al-Kattan A, Wichser A, Zuin S et al (2014) Behavior of TiO2 released from Nano-TiO2-containing paint and comparison to pristine nano-TiO2. Environ Sci Technol 48:6710–6718. doi:10.1021/es5006219
Arvidsson R, Molander S, Sandén BA, Hassellöv M (2011) Challenges in exposure modeling of nanoparticles in aquatic environments. Hum Ecol Risk Assess 17:245–262
ASTM E729-96, 2004 (2007) Standard guide for conducting acute toxicity testing on test materials with fishes, macroinvertebrates, and amphibians. ASTM International, West Conshohocken. doi:10.1520/E0729-96R07
Battin TJ, Kammer FV, Weilhartner A et al (2009) Nanostructured TiO2: transport behavior and effects on aquatic microbial communities under environmental conditions. Environ Sci Technol 43:8098–8104. doi:10.1021/es9017046
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Brunelli A, Pojana G, Callegaro S, Marcomini A (2013) Agglomeration and sedimentation of titanium dioxide nanoparticles (n-TiO2) in synthetic and real waters. J Nanopart Res 15:1–10
Buettner KM, Rinciog CI, Mylon SE (2010) Aggregation kinetics of cerium oxide nanoparticles in monovalent and divalent electrolytes. Colloid Surf A Physicochem Eng Asp 366:74–79. doi:10.1016/j.colsurfa.2010.05.024
Chen KL, Elimelech M (2006) Aggregation and deposition kinetics of fullerene (C60) nanoparticles. Langmuir 22:10994–11001
Chinnapongse SL, MacCuspie RI, Hackley VA (2011) Persistence of singly dispersed silver nanoparticles in natural freshwaters, synthetic seawater, and simulated estuarine waters. Sci Total Environ 409:2443–2450. doi:10.1016/j.scitotenv.2011.03.020
Chowdhury I, Duch MC, Mansukhani ND et al (2013) Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment. Environ Sci Technol 47:6288–6296
Dahirel V, Jardat M (2010) Effective interactions between charged nanoparticles in water: what is left from the DLVO theory? Curr Opin Colloid Interface Sci 15:2–7. doi:10.1016/j.cocis.2009.05.006
DeLoid G, Cohen JM, Darrah T et al (2014) Estimating the effective density of engineered nanomaterials for in vitro dosimetry. Nat Commun 5:3514. doi:10.1038/ncomms4514
Detloff T, Sobisch T, Lerche D (2007) Particle size distribution by space or time dependent extinction profiles obtained by analytical centrifugation (concentrated systems). Powder Technol 174:50–55
Erné B (2012) Sedimentation equilibria of colloidal dispersions in ultrathin glass capillaries. Dispers Lett 3:16–17
Farré M, Gajda-Schrantz K, Kantiani L et al (2009) Ecotoxicity and analysis of nanomaterials in the aquatic environment. Anal Bioanal Chem 393:81–95. doi:10.1007/s00216-008-2458-1
Gambinossi F, Mylon SE, Ferri JK (2015) Aggregation kinetics and colloidal stability of functionalized nanoparticles. Adv Colloid Interfac 222:332–349. doi:10.1016/j.cis.2014.07.015
Gottschalk F, Sonderer T, Scholz RW, Nowack B (2010) Possibilities and limitations of modeling environmental exposure to engineered nanomaterials by probabilistic: material flow analysis. Environ Toxicol Chem 29:1036–1048
Hartmann NB, Skjolding LM, Hansen SF et al (2014) Environmental fate and behaviour of nanomaterials. The Danish Environmental Protection Agency, Copenhagen
Hotze EM, Phenrat T, Lowry GV (2010) Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J Environ Qual 39:1909–1924
Hunter RJ (1988) Zeta potential in colloidal science—principles and applications, 3rd edn. Academic Press Limited, London
Jarvie HP, Al-Obaidi H, King SM et al (2009) Fate of silica nanoparticles in simulated primary wastewater treatment. Environ Sci Technol 43:8622–8628. doi:10.1021/es901399q
Jiang J, Oberdörster G, Biswas P (2009) Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res 11:77–89. doi:10.1007/s11051-008-9446-4
Keller AA, Wang H, Zhou D et al (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44:1962–1967
Kennedy Alan J, Hull MS, Steevens JA et al (2008) Factors influencing the partitioning and toxicity of nanotubes in the aquatic environment. Environ Toxicol Chem 27:1932–1941. doi:10.1897/07-624.1
Krause B, Mende M, Potschke P et al (2010) Dispersability and particle size distribution of CNTs in an aqueous surfactant dispersion as a function of ultrasonic treatment time. Carbon 48:2746–2754. doi:10.1016/j.carbon.2010.04.002
Lerche D (2002) Dispersion stability and particle characterization by sedimentation kinetics in a centrifugal field. J Dispers Sci Technol 23:699–709. doi:10.1081/DIS-120015373
Liu J, Hurt RH (2010) Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci Technol 44:2169–2175. doi:10.1021/es9035557
Liu HH, Surawanvijit S, Rallo R et al (2011a) Analysis of nanoparticle agglomeration in aqueous suspensions via constant-number monte carlo simulation. Environ Sci Technol 45:9284–9292. doi:10.1021/es202134p
Liu X, Wazne M, Chou T et al (2011b) Influence of Ca2+ and Suwannee River humic acid on aggregation of silicon nanoparticles in aqueous media. Water Res 45:105–112. doi:10.1016/j.watres.2010.08.022
Liu J, Legros S, Ma G et al (2012) Influence of surface functionalization and particle size on the aggregation kinetics of engineered nanoparticles. Chemosphere 87:918–924
Loux NT, Su YS, Hassan SM (2011) Issues in assessing environmental exposures to manufactured nanomaterials. Int J Environ Res Public Health 8:3562–3578
Lowry GV, Hotze EM, Bernhardt ES et al (2010) Environmental occurrences, behavior, fate, and ecological effects of nanomaterials: an introduction to the special series. J Environ Qual 39:1867–1874
Mueller NC, Nowack B (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42:4447–4453
Nowack B, Ranville JF, Diamond S et al (2012) Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ Toxicol Chem 31:50–59
Nowack B, Mueller N, Krug H, Wick P (2014) How to consider engineered nanomaterials in major accident regulations? Environ Sci Eur 26:2
OECD (1992) Guidelines for Testing of Chemicals No. 203. Fish, Acute Toxicity Test (Annex 2 Composition of the recommended reconstituted water). OECD, Paris
OECD (2006) Freshwater alga and cyanobacteria, growth ınhibition test. Organisation for Economic Co-Operation and Development, Paris
Pettitt ME, Lead JR (2013) Minimum physicochemical characterisation requirements for nanomaterial regulation. Environ Int 52:41–50
Phenrat T, Saleh N, Sirk K et al (2006) Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ Sci Technol 41:284–290. doi:10.1021/es061349a
Piccinno F, Gottschalk F, Seeger S, Nowack B (2012) Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res 14:1–11. doi:10.1007/s11051-012-1109-9
Praetorius A, Scheringer M, Hungerbühler K (2012) Development of environmental fate models for engineered nanoparticles—a case study of TiO2 nanoparticles in the Rhine river. Environ Sci Technol 46:6705–6713
Quik JTK, Lynch I, Hoecke KV et al (2010) Effect of natural organic matter on cerium dioxide nanoparticles settling in model fresh water. Chemosphere 81:711–715. doi:10.1016/j.chemosphere.2010.07.062
Quik JTK, Vonk JA, Hansen SF et al (2011) How to assess exposure of aquatic organisms to manufactured nanoparticles? Environ Int 37:1068–1077
Quik JTK, Stuart MC, Wouterse M et al (2012) Natural colloids are the dominant factor in the sedimentation of nanoparticles. Environ Toxicol Chem 31:1019–1022. doi:10.1002/etc.1783
Quik JTK, van De Meent D, Koelmans AA (2014a) Simplifying modeling of nanoparticle aggregation–sedimentation behavior in environmental systems: a theoretical analysis. Water Res 62:193–201. doi:10.1016/j.watres.2014.05.048
Quik JTK, Velzeboer I, Wouterse M et al (2014b) Heteroaggregation and sedimentation rates for nanomaterials in natural waters. Water Res 48:269–279. doi:10.1016/j.watres.2013.09.036
Ramsden CS, Henry TB, Handy RD (2013) Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquat Toxicol 126:404–413. doi:10.1016/j.aquatox.2012.08.021
Schwarzer H-C, Peukert W (2005) Prediction of aggregation kinetics based on surface properties of nanoparticles. Chem Eng Sci 60:11–25
Schwyzer I, Kaegi R, Sigg L et al (2012) Long-term colloidal stability of 10 carbon nanotube types in the absence/presence of humic acid and calcium. Environ Pollut 169:64–73
Sobisch T, Kuechler S, Uhl A (2010) Comprehensive characterization of medical nutrition—accelerated stability analysis and creaming velocity distribution. Dispers Lett 1:10–13
Sobisch T, Le Coeur C, Larue O et al (2012) Characterization of sedimentation and consolidation behaviour of kaolin suspensions in presence of dispersant. Dispers Lett 3:18–22
Suttiponparnit K, Jiang J, Sahu M et al (2011) Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res Lett 6:27
Takeuchi Y, Ida T, Kimura K (1997) Colloidal stability of gold nanoparticles in 2-propanol under laser irradiation. J Phys Chem B 101:1322–1327. doi:10.1021/jp963107a
Tiraferri A, Chen KL, Sethi R, Elimelech M (2008) Reduced aggregation and sedimentation of zero-valent iron nanoparticles in the presence of guar gum. J Colloid Interface Sci 324:71–79. doi:10.1016/j.jcis.2008.04.064
U.S. Environmental Protection Agency (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. EPA/812/R/02/012. Office of Water, Washington, DC
Van der Zande BMI, Dhont JKG, Bohmer MR, Philipse AP (2000) Colloidal dispersions of gold rods characterized by dynamic light scattering and electrophoresis. Langmuir 16:459–464
Van Koetsem F, Verstraete S, Van der Meeren P, Du Laing G (2015) Stability of engineered nanomaterials in complex aqueous matrices: settling behaviour of CeO2 nanoparticles in natural surface waters. Environ Res 142:207–214. doi:10.1016/j.envres.2015.06.028
Villarreal FD, Das GK, Abid A et al (2014) Sublethal effects of CuO nanoparticles on Mozambique tilapia (Oreochromis mossambicus) are modulated by environmental salinity. PLoS ONE 9:e88723. doi:10.1371/journal.pone.0088723
Wang F, Yao J, Chen H et al (2013) Sorption of humic acid to functionalized multi-walled carbon nanotubes. Environ Pollut 180:1–6. doi:10.1016/j.envpol.2013.04.035
Wiesner MR, Lowry GV, Jones KL et al (2009) Decreasing Uncertainties in assessing environmental exposure, risk, and ecological implications of nanomaterials†‡. Environ Sci Technol 43:6458–6462. doi:10.1021/es803621k
Zhang Y, Yang M, Portney N et al (2008) Zeta potential: a surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed Microdevices 10:321–328. doi:10.1007/s10544-007-9139-2
Zhang Y, Chen Y, Westerhoff P, Crittenden J (2009) Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res 43:4249–4257. doi:10.1016/j.watres.2009.06.005
Zhang W, Crittenden J, Li K, Chen Y (2012) Attachment efficiency of nanoparticle aggregation in aqueous dispersions: modeling and experimental validation. Environ Sci Technol 46:7054–7062
Acknowledgments
This research was partially founded by the European Commission within the Seventh Framework Program (FP7; SUN project - Grant Agreement n° 604305). The authors thankfully acknowledge VenetoNanotech for providing TEM images of MWCNT and Riccardo Cossi (Qi srl, Pomezia, Italy) and Andrea Scandella (University Ca’ Foscari of Venice) for their valuable technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brunelli, A., Zabeo, A., Semenzin, E. et al. Extrapolated long-term stability of titanium dioxide nanoparticles and multi-walled carbon nanotubes in artificial freshwater. J Nanopart Res 18, 113 (2016). https://doi.org/10.1007/s11051-016-3412-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3412-3