Abstract
Nanotechnology is widely used in several industrial and consumer sectors and has the potential to grow further and expand globally. An exploration of stakeholder (SH)’s perceptions is essential to ensuring that robust risk governance processes are in place for nanotechnology and nano-related products. In response, numerous studies have been conducted to investigate SH’s perceptions of nanotechnology and nano-related products over the past 15 years. To build on this work and to capture current perceptions across a wide panel of SHs, we conducted a multi-national and cross-sectoral SH study of awareness, perceptions and opinions regarding the use and potential impact on society and the environment of nanomaterials (NMs) and nano-related products, and SH’s expectations about risk governance. The study was conducted using both quantitative and qualitative inquiries and targeted more than 3000 SHs across different sectors in a total of 15 countries. Results showed a tendency towards more convergence of opinions amongst all the relevant SHs and the public respondents than in past studies. There was consensus on the crucial importance of having unbiased, scientific and trustable information regarding the potential impacts of NMs and nano-related products on the environment, health and safety. SHs were interested in having more internationally harmonised and robust regulation for NMs and nano-related products; improved scientific evidence on nanomaterial hazards, exposures and effects; as well as specific guidance on the safe use of NMs. Overall, this work provides an updated scenario of SHs’ perceptions regarding nanotechnology and nano-related products, underscoring the importance of including SH needs in effective risk governance strategies.
Similar content being viewed by others
Introduction
The use of nanomaterials (NMs) and nano-related products has become ubiquitous in society, with applications in diverse products and sectors that range from electronics, food and food packaging, transportation, energy, manufacturing, construction, medicine, water treatment and beyond (Woodrow Wilson Institute 2009; The Nanodatabase 2019). While the use of NMs and nano-related products may provide numerous benefits to diverse economic sectors, there are still significant uncertainties in terms of their potential impacts on the environment, health and safety (EHS), in spite of nearly two decades of EHS research (e.g. Prosafe 2017; OECD 2018; Warheit 2018). Furthermore, it has been well recognised that stakeholder (SH)’s perceptions are critical to understand and respond to in order to ensure the successful innovation and adoption of nanotechnology and nano-related products as well as to ensure that robust risk governance processes are in place (The Royal Society and The Royal Academy of Engineering 2004). This is particularly pertinent given previous cases involving other emerging technologies that have demonstrated the importance of understanding and incorporating SH perceptions into technology innovation processes (e.g. Tait 2001; Macnaghten 2016).
Numerous studies have been conducted over the past two decades to investigate various SH’s perceptions of nanotechnology and NMs (e.g. Sims Bainbridge 2002; Cobb and Macoubrie 2004; Siegrist et al. 2007a, 2007b; Kahan et al. 2008; Capon et al. 2015; Dijkstra and Critchley 2016; Ganesh Pillai and Bezbaruah 2017; Larsson et al. 2019). Across these studies, it has been demonstrated that perceptions of nanotechnology have been linked to the degree of knowledge and awareness about nanotechnology, potential benefits of its applications, cultural backgrounds, value differences as well as trust (Kahan et al. 2008; Cacciatore et al. 2011; Dijkstra and Critchley 2016; Ganesh Pillai and Bezbaruah 2017). In brief, the literature has demonstrated that the public generally has heard “little or nothing” about nanotechnology (Cobb and Macoubrie 2004; Priest et al. 2010; Gupta et al. 2015) and has had largely neutral or slightly optimistic opinions about nanotechnology (Cobb and Macoubrie 2004; Gaskell et al. 2005; Scheufele and Lewenstein 2005; Kahan et al. 2008; Satterfield et al. 2009), although there can be cross-cultural differences (Vandermoere et al. 2010; Dijkstra and Critchley 2016). Further, opinions about nanotechnology are still being formed and may likely be influenced by messaging concerning benefit-risk information (Satterfield et al. 2009; Priest et al. 2010; Parisi et al. 2015) and news on recently emerging cases of claimed health incidences (e.g. Bullis 2006; Kolosnjaj-Tabi et al. 2015; Fatkhutdinova et al. 2016; Zhao et al. 2018). In terms of risk-benefit comparisons, previous work showed that the potential benefits were considered to outweigh potential risks (Satterfield et al. 2009), although the authors considered that risk judgments were still malleable. Others have demonstrated that the provision of additional information that was framed in either a positive or negative light was able to influence respondents’ perceptions of the benefit-to-risk ratio of nanotechnology (Besley 2010). Further, other works have shown that potential benefits and risks were dependent on the application in which NMs or nano-related products were used (Cacciatore et al. 2011; Gupta et al. 2015). Amongst other findings, medical applications of nanotechnologies have had a higher benefit perception compared to consumer products that do not have a clear or obvious beneficial use (Cobb and Macoubrie 2004; Priest and Greenhalgh 2011; Gupta et al. 2015), and perceived risks were greater in food applications than in other consumer products (e.g. electronics, energy applications) (Siegrist et al. 2007a, 2007b; Grobe et al. 2008; Siegrist et al. 2008; Brown et al. 2015).
Developing robust risk governance and decision-making processes related to NMs that incorporate SH’s views continues to be an ongoing challenge today. Amongst other obstacles, SHs differ in their risk perception along the value chain of a nano-related product (Malsch et al. 2017).
As robust risk governance frameworks are still developing for NMs and to further build on this body of literature, we conducted a large SH study of the awareness, perceptions and opinions regarding the production, use and potential impact of NMs and nano-related products on the society. The work was conducted as part of the European Horizon 2020 project, caLIBRAte (grant agreement no. 686239), with the goal of developing a next-generation nano-risk governance framework aligning with SH’s needs.
Methodology
A multi-national, mixed-method approach was utilised in this study to understand the opinions and perspectives of the public respondents and influential SHs from industry, research organisations and policy-oriented organisations across Europe, covering 15 countries. We aimed specifically at understanding the SHs’ levels of awareness and concerns regarding potential risks for the health and the environment posed by the development and use of NMs and nano-related products and expectations for risk governance in nanotechnology (defined as all aspects related to the identification, management, evaluation and communication of risks; International Risk Governance Council (IRGC) 2017).
Leveraging work conducted in previous studies that investigated risk perception of nanotechnologies, we employed a combination of quantitative and qualitative methods in our inquiries to collect and assess SHs’ opinions. The diverse specific methods used are summarised in Table 1. The methods were selected depending on the scope of the SH dialogue and the SHs’ level of knowledge or being informed about NMs, nano-related products and/or nanotechnology. As shown below, for example, the online consumer survey collected quantitative information on public perceptions of the risks, while the focus groups provide information on risk perception and explain the preferences and views on diffusion and use of NMs and nano-related products in a more qualitative way.
The different methodologies applied to gather and analyse the data on risk perception and awareness and on issues related to risk communication, assessment and management in the field of nanotechnology are briefly described below.
Two-round Delphi study
A two-round Delphi study was undertaken in the period from January to August 2017 to systematically investigate the opinions of different SHs from research, industry, insurance companies, government agencies and non-governmental associations about their appraisal of the risks posed by NMs and nano-related products and how to best manage and communicate these risks. In general, a Delphi study is a method of structuring a group communication process amongst a panel of geographically dispersed participants, mainly experts in areas that are relevant to the consultation, to deal with a complex problem (Dewar and Friel 2001). The overall goal is to organise a debate, collect and synthesise opinions and achieve a degree of convergence on selected themes of exploratory, predictive and even normative nature (Adler and Ziglio 1996; Linstone and Turroff 2002). To perform a Delphi study, experts are first asked to complete a questionnaire on selected themes. The evaluations of the first survey are disclosed in a following round so that experts are given the opportunity to compare their personal opinions with those expressed by the other participants, to refine their points of view and give some recommendations on critical issues with regard to the debated topics. The exchange of opinions over several rounds has the advantage that feedback processes encourage participants to re-examine their own evaluation. As a rule, the spectrum of assessments is reduced, trends become clearer and a satisfactory convergence of opinions is reached.
Since the method was first proposed at the Rand Corporation (Dalkey 1969), variations of the Delphi method have evolved, in an effort to meet the specific needs of different decision makers (Linstone 1998). Delphi surveys are no longer simply about achieving consensus, and they are more often used to identify different clusters of opinions. In practice, modern Delphi surveys do not make much, if any, use of iterations of the questionnaire. These Delphi surveys employ only two rounds of survey, inviting a deepening of exploration in the second round rather than aiming for consensus of the group. Thus, an individual can express a distinctly different opinion to the group perspective. Moreover, recent approaches develop interactive forms, in which one or more workshops are staged between the two rounds to facilitate the final assessments. This implementation of the Delphi exercise was adopted for the present study where multiple perspectives of different SHs were recommended for decision-making. It means that the responses from a selected group of the participants could be extracted and analysed in isolation, without compromising the integrity of the results.
In the present two-round Delphi study, the initial questionnaire (see Online Resource 1) contained a total of 28 (mostly quantitative) questions covering issues specifically related to NMs and nano-related products such as awareness and risk perception, best practices in dealing with risk assessment/management and needs, priorities and expectations to a risk governance framework. An online platform was used to deliver questionnaires and collect data.
The first-round questionnaire was delivered to over 400 SHs in diverse European countries. These SHs were selected for participation based on their expertise and interest in risk governance aspects of NMs. After reaching out to these SHs, we sent two subsequent reminders to increase the response rate. The final response rate of SHs participating in our two-round Delphi study was approximately 25%, with a total of 97 participants from at least 14 European countries (some participants did not provide country of provenance). Study participants were able to answer the questionnaire anonymously. Nearly two thirds of all SHs chose to identify themselves according to a particular SH category (i.e. 69 participants), consisting of industry/business (38%), research (31%), policy makers/risk assessors/consultants (18%), civil society organizations (e.g. NGOs, advocacy groups) (10%) and insuranceFootnote 1 (3%).
Following analyses of the results from the first-round and the multi-SH workshop organised between the two rounds (see the section “Multi-SH workshop”), the findings were used to formulate the second questionnaire (see Online Resource 2), which included new, more qualitative and in-depth questions on the same themes treated in the first questionnaire, to enable further elaboration on the most salient aspects emerging from the first round. Forty-seven participants from 13 countries completed the second Delphi study round. Nearly all (i.e. 41 out of 47 participants) indicated their SH category with the following distributions: industry/business (49%), research (37%), policy makers/risk assessors/consultants (12%) and insurance (see footnote 1) (2%). Respondents were invited to read and get acquainted with the summarised opinions that had been expressed in the first round and were encouraged to express their opinions on the most debated topics and to make recommendations for developing an effective risk governance framework for NMs and nano-related products.
The data from the two online surveys were analysed qualitatively and quantitatively.
Multi-SH workshop
In addition to the two-round Delphi study, a SH workshop was organised in VeniceFootnote 2 (2017) and included 25 invited SHs from industry, insurance, consultancy services, policy and research from ten European countries. During the World Café, the results of the 1st round of the Delphi survey were presented, and afterwards, the SHs discussed needs, priorities and views on managing risks in nanotechnology. In addition, the SHs provided feedback on developing a risk governance framework for NMs. Using a World Café approach, groups of five participants at small round tables discussed questions (see Online Resource 3) on several issues relevant to nano-risk governance and wrote their opinions with pencils on the tablecloth. After about 25 min, the participants changed tables, took their ideas and shared them with the new table. One participant, the “host” or “facilitator” of the table, stayed seated and welcomed the new participants to another round of talks.
This interactive method allowed to exchange and then link the ideas of the participants and to draw a “network” of thoughts and perspectives to develop conclusions. In a joint final round of the SH workshop, the hosts of the tables presented the results, which were further discussed in a plenary session (www.theworldcafe.com).
Face-to-face interviews
Twelve interviews were conducted in Denmark and Sweden with occupational safety and health (OSH) managers from industry (n = 6) and academic institutions (n = 6). Heterogeneous (maximum variation) sampling (Palinkas et al. 2015) was used to recruit a variety of companies (e.g. manufacturers of materials and/or products, and testing and development of industry equipment) and institutes (e.g. traditional and within different technical fields) that worked with NMs. The interviews were conducted face-to-face and on site at each of the companies or institutions. The interviews occurred in 2016–2017, at which point, there was no nano-specific regulation. To foster consistency between the interviews, a semi-structured interview schedule was utilised (see the list of questions in Online Resource 4), which focused on five themes concerning NMs and nano-related products: risk comprehension, information gathering, actions, communication and compliance. The interviews were recorded, transcribed and then analysed in the NVivo 11 software program. In addition, relevant statements from the interviews were also coded according to one of five levels of safety culture maturity (Cooper 2018), from passive (ignorant of the risks) to reactive (respond only when things go wrong), active, proactive and exemplary (e.g. going beyond compliance). Further details on the methodology and a detailed analysis of the results can be found in Kirkegaard et al. (2019).
Focus groups
During organised focus group sessions, participants representing specific interests are encouraged to provide information and opinions on a specific topic in a discussion round (Henseling et al. 2006; Krueger and Casey 2008). Focus groups work out specific arguments and reflect them within a setting of several people (Benighaus and Benighaus 2012). The interaction and discussion in the focus groups help illustrate perceptions, perspectives and thinking of the different SH groups. It works well in combination with individual interviews or Delphi exercise.
In our study, a total of 6 focus groups were organised in 2017 and 2018 to explore and evaluate the perception and acceptance of nanotechnology from the public respondents (in terms of benefits and risks), the criteria for choosing and buying nano-enabled products and the information required to feel safe about their use. A list of the questions posed in the focus groups is reported in Online Resource 5.
All focus groups were conducted in the native languages of each country. In Germany, the participants were selected in a random sample. After contacting an initial 1000 citizens from the city of Stuttgart by letter, there were a total of 43 people who ended up participating in the four focus groups. In Spain, one focus group was organised in Bilbao, with seven participants selected from the employers of technological park by inviting them randomly. In Denmark, after placing an advertisement in supermarket and municipal websites in Copenhagen, there were a total of 7 focus group participants.
Overall, a statistical analysis and comparison of the focus group participants with the Eurostat statistical data on age and educational background (System 2011) from each of the participating countries revealed that the focus group participants were younger in age and had above-average educational level in each country than the statistical average.
Online consumer survey
In three European countries (Denmark, Germany, Spain), a large online consumer survey was conducted using a set of questions (see Online Resource 6) to elicit public perceptions on nanotechnologies. In total, there were 3101 participants who completed the online survey, including 1037 from Denmark, 1030 from Germany and 1034 from Spain. In these online consumer surveys, there was a good dispersion of different ages, genders and locations of the participants, and it is was also consistent with the Eurostat (System 2011) statistical data of the German, Spanish and Danish population.
Results
We present the main points according to the following: (1) risk perception of NMs and nano-related products according to the SH level of knowledge and/or awareness, (2) factors limiting the acceptance and diffusion of NMs and nano-related products into the market and (3) SH expectations in regard to nano-risk governance.
Risk perceptions and awareness of nanotechnology in different SH groups
The majority of the SHs involved in the Delphi consultation had a high level of knowledge on NMs. More specifically, 8% of all the respondents (N = 97) reported to have a basic level of knowledge, while 73% reported themselves as experts or skilled users. Figure 1 provides an overview of the level of knowledge on NM according to each SH groupFootnote 3. As expected, researchers are the most skilled category, with other SH groups adequately knowledgeable and informed. Some of the comments provided by the respondents indicated that they were aware of the opportunities offered by nanotechnologies for progress and innovation. In particular, the SHs from industry underlined “the enormous potential of nanotechnologies for competitiveness of industry”.
However, the opinion concerning the positive potentials of nanotechnologies is also associated with a general concern about the EHS risks and social and ethical aspects posed by NMs and nanotechnology development in all the application domains (Fig. 2). Noteworthy, it can be seen that the relative perceived risk pattern in the six main predefined domains (worker health, public health, animal health, economic aspects, ethical and social domains) is generally similar amongst all the SHs involved in the Delphi study, with workers and the environment being at the highest risk level (moderate to high). However, the civil society organizations (CSOs)’ SH group does stand out with generally higher perceived risks while policy makers/risk assessors/insurers have the lowest level of perceived risk. On a relative scale, all the SHs have relatively low perceived risk in regard to economic aspects. Still, it should be mentioned that researchers consider risks related to economic aspects to be at a similar level as the risk to public health.
In the course of the qualitative workshop interviews, the industry representatives confirmed that they consider the workers and the environment to have the highest risks in relation to NM production. In the qualitative interviews with individuals in research organisations and companies, the perspectives on risk perception varied substantially, ranging from perceiving risks as a “part of the job” to perceiving risks as manageable throughout the life cycle of NM and nano-related products (Kirkegaard et al. 2019).
According to the public respondents from the online consumer survey, participants agreed that the pros and cons of nanotechnology are balanced, and nanotechnology has a positive or neutral connotation for them with positive effect on life (Fig. 3). More than 50% of participants in all three countries (Denmark, Germany and Spain) perceived nanotechnology as a symbol of progress and an option for breakthrough in future technologies. However, they anticipate a high responsibility for future generations in all three countries, especially in the development of nanotechnologies.
The participants in the focus groups have indicated that nanotechnology should be a solution for technical and/or social problems and should support the development of society. Yet, there are some differences amongst the answers of respondents from the various countries regarding their perceptions on how well the advantages and disadvantages of NMs are balanced. Our study did not seek to address these inter-country differences in participants’ perceptions of risks/benefits.
During the focus group interviews, participants assessed nanotechnology based on their own experience and how they got in contact with it. After discussion about its advantages and disadvantages, they developed a more critical opinion about the use of nanotechnology, expressing concern about the safety of nanotechnology for society, the environment and economy as well as individual and family health (public and workers’ health).
By combining the results from the different consultations, it appears that the level of risk perception by the different groups of SHs is only to a certain extent related to the level of knowledge about nanotechnology, NM and nano-related products (Table 2). Our results also showed that there was significant awareness about potential risks for the EHS during research/development/fabrication of NMs and nano-related products (Fig. 2, Table 2), which was also confirmed by the outcomes of the SH workshop and individual SH interviews in industry and academia. At the same time, the public respondents are concerned about EHS risks (Fig. 4, Table 2) but appreciate on average the positive connotations of nanotechnologies (Fig. 3). It should be noted that the perceived social and ethical risks are greater to CSOs and public respondents than to the other three categories of SHs.
Although some caution is required in interpreting the results in Table 2, compared to earlier studies (Grobe et al. 2008), neither a clearly positive correlation nor a negative correlation between knowledge on NMs and risk concern is observed.
In this context, it is important to consider that the different SH groups and the public respondents do not perceive the same level of risk in all nanotechnology applications. In fact, a considerable difference is observed across different sector applications (Fig. 5). The respondents representing industry consider the highest risks associated with the use of NMs in food, agri-food, cosmetics and packaging, and chemicals and materials, but the level of risk perception is somewhat lower than that expressed by the CSOs’ representatives who also consider applications in textiles and the environment potentially risky. Academic researchers are most concerned about nanotechnology in chemicals and materials followed by the use of nanotechnology in application sectors related to energy, electronics, optics and information and communication technologies (ICTs).
Delphi survey. Perception by the different categories of SHs of the risks potentially posed by production use and disposal of NMs and nano-related products in different application domains and market sectors on a scale of 1 (very low risk) to 5 (very high risk). (NIndustry = 29; NResearch = 21; NPolicy = 13; NCSOs = 6)
The perceived sector application risk pattern reported by the CSOs is in good agreement (with a few exceptions) with the outcomes of the focus groups and online consumer survey (Fig. 6), where the main concerns are about nano-related products that people may come into direct physical contact with (absorbed through the skin, inhaled, ingested, etc.).
It is interesting to see that the highest acceptance of nano-related products from the public respondents refers to IT/electronics with miniaturised electronic components and to medical products which potentially provide new forms of detection and treatment of disease.
In summary, although some differentiation emerges across the SH categories and the public respondents, our results provide evidence that medium to moderately high risks are perceived in all application domains and market sectors (see Figs. 5 and 6), but at the same time, potential benefits of nanotechnology are recognised and appraised by many (Fig. 4).
Although the development of nanotechnology is considered as an opportunity for improving performances of products and systems, the perceived uncertainty on their potential risks is seen to influence to a certain extent and limit their purchase. This point was sustained by a good fraction of the participants in the online consumer survey (Fig. 7), focus groups and Delphi study. Results are reported in Table 3 where a rather high level of convergence of views between all the SHs and the public respondents is observed.
Once again, policy makers/risk assessors/insurers show a lower concern about the effect of risk perception on the market than the other categories of SHs.
Factors limiting acceptance and adoption of NMs
In this section, we present perspectives from all the SHs on the factors that still hamper a wider acceptance and adoption of NMs and nano-related products. The participants in our consultation activities have clearly indicated that one of the most important factors for limiting the diffusion of nanotechnology into the market is a certain lack of dialogue and specific knowledge/education initiatives. Moreover, the SHs perceive an often misleading (or biased) information on NMs and nano-related products that may create a sense of suspicion in the entrepreneurs, end-users and consumers and make it difficult to evaluate the risk/benefit ratio with respect to the use of more conventional materials/products. In this respect, SHs participating in the Delphi study underlined that: “From the consumer side, I think that a general factor is the lack of scientific education and the influence of certain media attitude”. Thus, “Normal customers mostly know simple black and white, and cannot differentiate between various scenarios of grey”.
It was pointed out by many different SHs and the participants in the consumer survey that greater trust of the potential users could be gained through transparent and exhaustive information on safe use, storage and disposal of NMs and nano-related products.
Industry representatives are also concerned about existing uncertainties and insufficient knowledge about worker and consumer exposure, hazard and life cycle assessment. One of the industry representatives underlined that: “From the business side, there is the complete lack of knowledge about NMs: most SMEs [small and medium sized enterprises] do not know what they are and how they can be used. Therefore, once confronted with the possibility, entrepreneurs stay away from the unknown, the potentially not safe solutions, and rely on more traditional innovative materials…”.
Further scientific research on NMs is asked for, in order to decrease risks and increase confidence in their use.
A strong request emerging from both the online survey (public respondents) and the focus groups is the need for a clearer: “balance between pros and cons of nanotechnology”, which takes into account the impact of NMs and nano-related products on (human and animal) health and safety and the environment. To this respect, a telling statement from a researcher (Delphi survey) is that: “NMs used to cure cancer should be permitted, even if these NMs may induce some negative effects to human health and the environment”. This concept is broadened by one of the industry representatives: “Potential risks of the innovation must always be balanced against the risk of not realizing an innovation”.
In this context, CSOs’ representatives pointed out the need to set up an evaluation system of the risks posed by specific NMs and nano-related products, which must be based on principles such as openness, full independence and transparency, and thus become trustable for consumers. Industries should disclose their data to inform this system.
The participants in the focus groups and online consumer survey stressed the importance of having access to public product registers containing information about the ingredients (including nano-ingredients) of all products.
Given that labelling about the presence of nano-ingredients is already mandatory for food and cosmetic products, the opportunity to extend this requirement to all sectors is still up to debate. A very high percentage (95%) of the consumers participating in the online consumer survey think that if a product contains NMs, they have to be highlighted in the list of ingredients on the label. On the same way, some SHs participating in the Delphi study think that: “nano-labelling could give a wrong message that all nano-products are dangerous since consumers are not aware or competent to distinguish between sensible and non-sensible applications”.
In the course of the consultation activities, the SHs underlined that the perception of uncertainty in the safety of NMs and nano-related products is enhanced by some insufficiency and incoherence of the legislation all over Europe and by the lack of universally accepted definition of the term nanomaterial, of clear nanomaterial labelling/registration procedures and of specific guidelines for implementing current regulations.
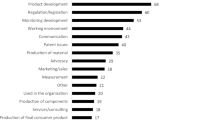
In particular, the existing normative framework is considered, to some extent, not fully adequate for risk governance of nanotechnology, above all by the CSOs’ representatives and by researchers participating in the Delphi survey (see Fig. 8). A more moderate criticism stems from the policy makers/regulators.
In summary, in the course of our consultation activities, it was recommended by the relevant SHs and the public respondents to develop the following actions with the aim to foster the diffusion of NMs and nano-related products into the market:
Organise more specific knowledge and education initiatives addressed to the public respondents.
Provide clear information to consumers on how to safely handle and dispose of NMs and nano-related products.
Ensure transparency in the communication from producers to consumers.
Promote public dialogue about the risks-to-benefit ratio.
Sustain scientific progress in assessing the real risks of NMs and nano-related products (including the toxicological and biological properties).
Improve the existing normative frameworks for NMs and nano-related products.
SH expectations on nano-risk governance
In the following section, we focus on the expectations expressed by the SHs in the course of our inquiries for viable risk assessment, communication and management of nanotechnology.
The first point concerns the generally acknowledged need to improve the existing normative framework for NMs and nano-related products. When asked in the course of the Delphi survey about the policy options to better integrate risks related to NMs and nano-related products in the existing regulation, different opinions emerged across the various categories of SHs. Policy makers/risk assessors/insurers were more in favour of inclusion of specific requirements for NMs in the existing “horizontal” regulations (e.g. REACH, CLP) whereas CSOs’ representatives insisted on the need for specific requirements in existing “vertical/product” regulations (e.g. cosmetics, medical devices, biocides) or even on the development of new regulation specifically designed for NMs and nano-related products. Some interest was expressed for the development and use of voluntary tools for risk governance; however, one of the SHs underlined that: “Voluntary tools need to be accepted by regulators and competent authorities, as well as seen as trustworthy by consumers”, otherwise they are not useful.
Although there are some divergent opinions on the development of the new regulatory frameworks, there is a very high consensus amongst all the SHs on the importance of risk assessment procedures for safe production, handling and use of NMs and nano-related products (see Fig. 9).
A respondent from industry commented that: “I believe we need to have good methods to assess and also to communicate risks. There will be more confidence in the assessment if it is systematic and transparent, and a good assessment procedure provides the structure and data for clear communication about the approach and the findings”.
Some divergences appear, however, on the most appropriate stage(s) of the value chain of where to address the assessment of the potential risks posed by the development of NMs and nano-related products.
In the opinion of the researchers participating in the Delphi survey, risk assessments should preferably be performed at the basic research/proof of concept stages to anticipate the potential risks and consider the risk mitigation plan: “Earlier done risk assessments reduce the potential of late development failures which may be costly”.
The importance of involving consumers and consumer organisations in the risk assessment procedures is stressed by many. In particular, more than 88% of the consumers participating in the online consumer survey support the idea of the early involvement of consumer organisations in the risk assessment of nano-related products, though only about 69% recommend that the consumers themselves should be involved in the following development stages.
Some industry respondents, however, are very concerned about the possibility that risk assessments performed: “at the very early stage of the innovation, could lead to a premature end of the research”. This is due to the high degree of uncertainty: “on how the final product will look like, and as well on its potential risks”. It was added that: “Risk assessments should not hinder the research process”. These SHs suggested that: “the best step is at the prototype phase”, or even later “in the go to development phase (that, however, is often the most expensive)”.
As opposed to this point of view, some industry representatives and researchers think that the most effective approach, if there are enough resources, would be to carry out risk assessment procedures all along the value chain, possibly through a tiered approach.
Due to the complexity of risk management procedures for safe production, use and disposal of NMs and nano-related products, the availability of decision support (DS) tools is judged in a positive way by all the SHs, above all by the industry representatives. These tools should support learning and orientation in decision-making on how to ensure safety of the research and innovation processes, including identification of safe by design approaches and definition of priorities for risk assessment and management of NMs and nano-related products.
Presently, the main barriers to the adoption of these tools were indicated as the complexity in their use, insufficient information on the features/capabilities of these tools and the difficulty in providing input data.
The DS tools should be linked to reliable and exhaustive sources of data for risk assessment, and possibly support risk-benefit evaluation. Availability of benchmarks (e.g. bulk materials) would also be welcomed.
In order to be widely accepted by SHs, these tools should be aligned with and support compliance with existing regulatory frameworks.
The possible impact of all these findings on the development of a viable risk governance framework for NMs and nano-related products is presented and discussed in the next paragraph.
Discussion
This study provides critical insights on current SH opinions on risk perception and risk governance of NMs and nano-related products using a mixed method approach. We analysed the results according to SH groups as well as their level of knowledge and awareness of NMs, nano-related products and nanotechnologies more generally. In addition, this study leverages the outcomes from a number of previous, generally more focused, studies on SH opinions undertaken in the last decade, and provides an opportunity to confirm or observe changes in attitudes and perceptions of SHs since earlier studies were conducted.
We found that the majority of the study participants regard nanotechnologies as a symbol of innovation and progress and perceive that their use could imply both benefits and risks. The highest risks perceived are related to the health and safety of workers, consumers and public health and impact on the environment in the use of NMs. In addition, we found that informed SHs have a medium-to-high perception of the risks of NM, and that they consider existing uncertainties on risks of NM as a major barrier for their market exploitation. They strongly believe further research is needed on evidence-based approaches for risk analysis.
We also found that SHs with more limited awareness of nanotechnologies, including the public respondents’ category, generally had a more of a positive attitude towards the development of nanotechnologies in most sectors. The highest appraisal and acceptance of nano-related products from the public respondents refers to electronics/IT with miniaturised electronic components and to promising medical products. Other scholars confirmed these findings as part of their research (Cobb and Macoubrie 2004; Priest and Greenhalgh 2011; Gupta et al. 2015). At the same time, the risk perception of NMs and nano-related products was greater for products that could get in direct contact with the body (e.g. cosmetics, food, agri-food). Potential intake of NMs is seen as alarming, and nano-based food products are seen as non-genuine and artificial. These findings confirm previous studies as, for example, those reported in the literature (Siegrist et al. 2007a, 2007b; Grobe et al. 2008; Siegrist et al. 2008; Ferdinand et al. 2013; Brown et al. 2015).
Furthermore, the majority of SHs (including the public respondents) were aware of and agree on existing gaps and barriers for risk governance of NMs, thus pointing towards a clear need for more robust and effective approaches to risk governance. In terms of the adequacy of existing regulatory frameworks, many SHs considered that these are not fully satisfactory, or at least not sufficiently comprehensive, to ensure control and oversight of production, use and end-of-life of NMs and nano-related products. The lack of harmonised approaches across regulatory domains, uncertainties in the implementation of regulation and also limited awareness on most recent developments in regulation (as NMs are now explicitly considered in several EU regulations) are all elements that contribute to this perspective.
One interesting and perhaps novel outcome of the present work is that our study revealed a tendency towards a convergence of opinions between different SHs (industry representatives, researchers, policy makers, regulators, insurers, society representatives and the public respondents) and a positive attitude towards future developments of risk governance of nanotechnologies. In fact, across our study, there were no strongly controversial issues or opposing views between informed SHs and the public respondents. This is in contrast with previous studies, in which more polarisation of debate was reported (Einsiedel 2005; Grobe et al. 2008; Ferdinand et al. 2013).
This study also identified some elements that strongly influence SH risk perception, with implications for the acceptance of NMs and nano-related products. For instance, given a specific product, or research and innovation value chain, risk analysis should take into account predefined conditions such as the sector and type of application concerned, the risk domains (e.g. workers, consumer health, end-of-life of product(s)), regulatory domains as well as any prior knowledge or background conditions in terms of public opinions and media coverage. Other key factors for risk governance, on which it is possible and requested to intervene, include the level of awareness or knowledge of SHs, the actual or perceived risk-benefit ratio of the specific product concerned and the quality, reliability and ease of understanding of available information such as, e.g. EHS and NM characterisation data, safety procedures and product information.
Based on these findings, we consider these aspects should deserve greater attention and should be taken into account by policy makers, research and industry representatives, using a research and innovation value chain perspective, considering the role, influence and interaction of different SHs on risk governance issues in each of the product development phases. While previous studies have emphasised the public respondents (i.e. business to consumer interactions), our analysis reveals that there is a need for improving risk communication also between and towards informed SHs including research-to-business SHs and business-to-business SH interactions. We believe that increased agreement and confidence of these actors is a prerequisite to inform and shape public opinion on issues related to risk governance of nanotechnologies.
Further development of the traditional evidence-based approach for risk assessment is seen by all SHs as essential. This includes improving the knowledge base, tools, guidelines and procedures for the characterisation and testing of NMs. The need to consider multi-parametric approaches and to address uncertainties in long-term impacts of NMs on public health and the environment is an aspect that has been emphasised by SHs. However, results of our study seem to suggest there is also a need to accompany the conventional science and evidence-based approach with novel risk governance approaches in order to address the complexity, as well as the pervasive and dynamic character of nanotechnology development. Therefore, harmonisation of practices and procedures within and across regulatory domains (and countries) should be promoted, to overcome current ambiguities and lack of guidance for the implementation of regulation, including issues of definition of NMs. This might require strengthening the cooperation between technology developers and regulators in order to deal with novel applications of nanotechnologies, which could also require specific or unexpected control and oversight approaches. In line with on-going activities in EU-funded projects on risk governance of nanomaterials, we recommend that a specific system or mechanism should be put in place to ensure access to reliable information and guidance on safe handling, use and disposal of NMs and nano-related product all along the research and innovation value chain, from the producers to the consumers. Building off of this need for reliable information and guidance, there is also a need for an evaluation system of the risks and benefits posed by specific NMs and related products, which must be based on principles such as openness, independence and transparency and thus become trustable for SHs. The type of NM, application sector, use scenarios along the life cycle, risks and benefits and customer and market segments should be considered in such an evaluation system. To underpin reliable information dissemination, there is also a need for more effective and harmonised communication efforts to achieve greater legitimacy, trust and trustworthiness amongst all the actors involved in developing, producing, regulating and selling an innovative nano-related product.
Concluding remarks
The study obtained information on SH perceptions of NMs and nano-related products in order to better inform and develop risk governance frameworks for NMs. These data are leveraged within the research project caLIBRAte in order to prioritise developments for a first nano-risk governance portal underpinned with nano-risk governance guidance and models for risk-screening, qualitative and quantitative risk assessment and management, which have been tested and approved in the project to improve trust in decision-making and risk communication. Recently, started H2020 governance research projects (Gov4Nano, NanoRigo and RiskGone) are now working to develop a broader risk governance framework, including better the safe-by-design concept, to connect and interlink data, experiences and competences across research areas, regulatory domains and SHs.
Notes
In the analysis of the answers to the Delphi questionnaires, the contributions from insurers are incorporated into the category of policy makers/risk assessors/consultants.
“From nano risk management to innovation governance: Developing state of the art, reliable and trustable, governance models and tools for nanomaterials” March 2–3, 2017, Venice, Italy
Not all the SHs participating in the Delphi survey declared the category to which they belong and contribute to the graphs in Fig. 1.
References
Adler M, Ziglio E (1996) Gazing into the Oracle: the Delphi method and its application to social policy and public health. Jessica Kingsley, London
Benighaus, C. and L. Benighaus (2012). Moderation, Gesprächsaufbau und Dynamik in Fokusgruppen. Fokusgruppen in der empirischen Sozialwissenschaft. M. Schulz, B. Mack and O. Renn. Berlin, VS Verlag für Sozialwissenschaften: 111–132.
Besley J (2010) Current research on public perceptions of nanotechnology. Emerging Health Threats Journal 3(1):7098
Brown J, Fatehi L, Kuzma J (2015) Altruism and skepticism in public attitudes toward food nanotechnologies. Journal of Nanoparticle Research 17(3):1–31
Bullis, K. (2006). “Nano” safety recall: a product touted as “nano” has hospitalized six German consumers, prompting more warnings over the dangers of nanomaterials. MIT Technology Review.
Cacciatore MA, Scheufele DA, Corley EA (2011) From enabling technology to applications: the evolution of risk perceptions about nanotechnology. Public Understanding of Science 20(3):385–404
Capon A, Gillespie J, Rolfe M, Smith W (2015) Perceptions of risk from nanotechnologies and trust in stakeholders: a cross sectional study of public, academic, government and business attitudes. BMC Public Health 15(1):424
Cobb MD, Macoubrie J (2004) Public perceptions about nanotechnology: risks, benefits and trust. Journal of Nanoparticle Research 6(4):395–405
Cooper, M. D. (2018). The safety culture construct: theory and practice. Safety cultures, safety models: taking stock and moving forward. C. Gilbert, B. Journe, H. Laroche and C. Bieder. Cham, Springer International Publishing: 47–61.
Dalkey NC (1969) The Delphi method: an experimental study of group opinion. Santa Monica, CA, Rand
Dewar JS, Friel JA (2001) Delphi method. Springer Science & Business Media, Berlin
Dijkstra AM, Critchley CR (2016) Nanotechnology in Dutch science cafés: public risk perceptions contextualised. Public Understanding of Science 25(1):71–87
Einsiedel E (2005) In the public eye: the early landscape of nanotechnologies among Canadian and US Publics. Journal of Nanotechnology Online 1
Fatkhutdinova LM, Khaliullin TO, Vasil’yeva OL, Zalyalov RR, Mustafin IG, Kisin ER, Birch ME, Yanamala N, Shvedova AA (2016) Fibrosis biomarkers in workers exposed to MWCNTs. Toxicology and Applied Pharmacology 299:125–131
Ferdinand, J.-P., M. Gossen, G. Scholl and B. Holzhauer (2013). Nanoview—Einflussfaktoren auf die Wahrnehmung der Nanotechnologien und zielgruppenspezifische Risikokommunikationsstrategien, Bundesinstitut für Risikobewertung.
Ganesh Pillai R, Bezbaruah AN (2017) Perceptions and attitude effects on nanotechnology acceptance: an exploratory framework. Journal of Nanoparticle Research 19(2):41
Gaskell G, Eyck TT, Jackson J, Veltri G (2005) Imagining nanotechnology: cultural support for technological innovation in Europe and the United States. Public Understanding of Science 14(1):81–90
Grobe, A., O. Renn and A. Jaeger (2008). Risk governance of nanotechnology applications in food and cosmetics. International Risk Governance Council (IRGC).
Gupta N, Fischer ARH, Frewer LJ (2015) Ethics, risk and benefits associated with different applications of nanotechnology: a comparison of expert and consumer perceptions of drivers of societal acceptance. NanoEthics 9(2):93–108
Henseling C, Hahn T, Nolting K (2006) Die Fokusgruppen-Methode als Instrument in der Umwelt- und Nachhaltigkeitsforschung. Berlin, Institute for Futures Studies and Technology Assessment 48
International Risk Governance Council (IRGC) (2017) Introduction to the IRGC risk governance framework. Lausanne, Switzerland, IRGC 50
Kahan DM, Braman D, Slovic P, Gastil J, Cohen G (2008) Cultural cognition of the risks and benefits of nanotechnology. Nature Nanotechnology 4:87
Kirkegaard, M. L., P. Kines and K. A. Jensen (2019). Safety culture, risk perception and handling of nanomaterial risks in academia and industry—case studies. Annals of Work Exposures and Health (Submitted).
Kolosnjaj-Tabi J, Just J, Hartman KB, Laoudi Y, Boudjemaa S, Alloyeau D, Szwarc H, Wilson LJ, Moussa F (2015) Anthropogenic carbon nanotubes found in the airways of Parisian children. EBioMedicine 2(11):1697–1704
Krueger RA, Casey MA (2008) Focus groups: a practical guide for applied research. Sage, Thousand Oaks
Larsson S, Jansson M, Boholm Å (2019) Expert stakeholders’ perception of nanotechnology: risk, benefit, knowledge, and regulation. Journal of Nanoparticle Research 21(3):57
Linstone HA (1998) Multiple perspectives revisited. Orlando, CA, USA, IAMOT
Linstone, H. A. and M. Turroff (2002). The Delphi method: techniques and applications. Published online: https://web.njit.edu/~turoff/pubs/delphibook/delphibook.pdf, ©2002 Murray Turoff and Harold A. Linstone
Macnaghten P (2016) Responsible innovation and the reshaping of existing technological trajectories: the hard case of genetically modified crops. Journal of Responsible Innovation 3(3):282–289
Malsch I, Subramanian V, Semenzin E, Zabeo A, Hristozov D, Mullins M, Murphy F, Linkov I, Marcomini A (2017) Comparing mental models of prospective users of the sustainable nanotechnology decision support system. Environment Systems and Decisions 37(4):465–483
OECD (2018). Investigating the different types of risk assessments of manufactured nanomaterials: identifying tools available for risk management measures and uncertainties driving nano-specific data needs. Series on the Safety of Manufactured Nanomaterials Organisation for Economic Co-operation and Development. No. 88.
Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K (2015) Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health 42(5):533–544
Parisi C, Vigani M, Rodríguez-Cerezo E (2015) Agricultural nanotechnologies: what are the current possibilities? Nano Today 10(2):124–127
Priest S, Greenhalgh T, Kramer V (2010) Risk perceptions starting to shift? U.S. citizens are forming opinions about nanotechnology. Journal of Nanoparticle Research 12(1):11–20
Priest SH, Greenhalgh T (2011) Nanotechnology as an experiment in democracy: how do citizens form opinions about technology and policy? Journal of Nanoparticle Research 13(4):1521–1531
Prosafe (2017). The ProSafe White Paper: Towards a more effective and efficient governance and regulation of nanomaterials: 1–46.
Satterfield T, Kandlikar M, Beaudrie CEH, Conti J, Herr Harthorn B (2009) Anticipating the perceived risk of nanotechnologies. Nature Nanotechnology 4:752
Scheufele DA, Lewenstein BV (2005) The public and nanotechnology: how citizens make sense of emerging technologies. Journal of Nanoparticle Research 7(6):659–667
Siegrist M, Cousin ME, Kastenholz H, Wiek A (2007a) Public acceptance of nanotechnology foods and food packaging: the influence of affect and trust. Appetite 49(2):459–466
Siegrist M, Keller C, Kastenholz H, Frey S, Wiek A (2007b) Laypeople’s and experts’ perception of nanotechnology hazards. Risk Analysis 27(1):59–69
Siegrist M, Stampfli N, Kastenholz H, Keller C (2008) Perceived risks and perceived benefits of different nanotechnology foods and nanotechnology food packaging. Appetite 51(2):283–290
Bainbridge WS (2002) Public attitudes toward nanotechnology. Journal of Nanoparticle Research 4(6):561–570
System, E. S. (2011). Eurostat census. https://urldefense.proofpoint.com/v2/url?u=https-3A__ec.europa.eu_CensusHub2_query.do-3Fstep-3DselectHyperCube-26qhc-3Dfalse&d=DwIGaQ&c=vh6FgFnduejNhPPD0fl_yRaSfZy8CWbWnIf4XJhSqx8&r=F8jRe85hu55p0hrOAPRTlXqBWmdi_A5USzQxvJXlzL2tJvyEWDgfrhKJOIzcdlym&m=CCw_d0AT2RcqnJlaVrmnHXf8WRyuLsaHS94Kh9HCToo&s=-n7cHuKBIkmru_FI2WCLE4G9oKRPghKIQOna0tb1uUQ&e= .
Tait J (2001) More Faust than Frankenstein: the European debate about the precautionary principle and risk regulation for genetically modified crops. Journal of Risk Research 4(2):175–189
The Nanodatabase (2019) The nanodatabase. The Ecological Council, Danish Consumer Council, DTU Environment
The Royal Society and The Royal Academy of Engineering (2004) Nanoscience and nanotechnologies: opportunities and uncertainties. The Royal Society, Plymouth, UK, pp 1–116
Vandermoere F, Blanchemanche S, Bieberstein A, Marette S, Roosen J (2010) The morality of attitudes toward nanotechnology: about God, techno-scientific progress, and interfering with nature. Journal of Nanoparticle Research 12(2):373–381
Warheit, D. B. (2018). Hazard and risk assessment strategies for nanoparticle exposures: how far have we come in the past 10 years? F1000Research 7: 376.
Woodrow Wilson Institute. (2009). Consumer products inventory. The project on emerging nanotechnologies.
Zhao L, Zhu Y, Chen Z, Xu H, Zhou J, Tang S, Xu Z, Kong F, Li X, Zhang Y, Li X, Zhang J, Jia G (2018) Cardiopulmonary effects induced by occupational exposure to titanium dioxide nanoparticles. Nanotoxicology 12(2):169–184
Acknowledgements
The authors gratefully acknowledge support for this work through diverse funding mechanisms.
This article is part of the caLIBRAte project funded by the European Union’s Horizon 2020 research and innovation program under grant agreement no. 686239.
In particular, KG acknowledges the Game-Changing Research Incentive Program (GRIP) funded through the North Carolina State University Office of Research, Innovation, and Economic Development, RTI International, and the Kenan Institute for Engineering, Science and Technology, as well as the Research Triangle Nanotechnology Network (RTNN)—a site in the US National Nanotechnology Coordinated Infrastructure (NNCI).
The authors wish to thank Elvio Mantovani (Airi, IT) and Ludger Benighaus (Dialogik, DE) for useful and stimulating suggestion and comments in all phases of our study, Marie Louise Kirkegaard (Danish Technological University, DK) for her valuable contribution in conducting and analysing the interviews and questionnaires in Denmark and Isabel Rodriguez-Llopis (Gaiker, ES) and her team for conducting the focus group in Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human participants and informed consent
Informed consent was obtained by participants of the interviews, focus groups and surveys carried out for this research. Collection of personal data havebeen performed in compliance with the EU General Data Protection Regulation and relevant national laws
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Porcari, A., Borsella, E., Benighaus, C. et al. From risk perception to risk governance in nanotechnology: a multi-stakeholder study. J Nanopart Res 21, 245 (2019). https://doi.org/10.1007/s11051-019-4689-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-019-4689-9