Abstract
The thermal condensation of formamide in the presence of mineral borates is reported. The products afforded are precursors of nucleic acids, amino acids derivatives and carboxylic acids. The efficiency and the selectivity of the reaction was studied in relation to the elemental composition of the 18 minerals analyzed. The possibility of synthesizing at the same time building blocks of both genetic and metabolic apparatuses, along with the production of amino acids, highlights the interest of the formamide/borate system in prebiotic chemistry.
Similar content being viewed by others
Introduction
The role of borate minerals in the synthesis of sugar from formaldehyde and glycolaldehyde is receiving increasing attention. Pivotal studies showed that boric acid (H3BO3) and mineral borates ulexite [NaCaB5O6(OH)6 ∙ 5H2O], kernite [Na2B4O6(OH)2∙ 3H2O] and colemanite (CaB3O4(OH)3∙ H2O) catalyze the formation and stabilization of pentoses in the cyclic furanose form (Prieur 2001; Ricardo et al. 2004; Scorei and Cimpoiaşu 2006). In particular, the stabilization of ribose, one of the few sugars supporting the Watson-Crick molecular recognition processes essential for Darwinian evolution, was observed. Due to the high electronic deficiency of boron, borate forms complexes with the vicinal 1,2-diol moiety in key intermediates of the formose condensation sequence leading to sugars, such as glyceraldehyde. These complexes provide selectivity to the reaction, preventing the nucleophilic character of glyceraldehyde, while retaining its electrophilicity towards enediolate glycolaldehyde condensing units. Moreover, at the end of the reaction, borate yields a complex with ribose which is more stable than ribose itself. Among the chemical precursors studied in prebiotic chemistry, formamide (NH2CHO) provides a unitary framework for the synthesis of nucleic acids precursors, amino acids, and amino-sugar derivatives under simple experimental conditions compatible with the primitive Earth (Saladino et al. 2004; Saladino et al. 2006; Saladino et al. 2009).
A possible relationship between formamide and borate minerals has been discussed by Benner et al. (2006) in the context of the “water paradox” stating that polymerization of biomolecules requiring release of water are, in water, thermodynamically disfavoured. During dry-wet cycles presumably fostering the formation of oligonucleotides, two processes can occur: on one hand the formation of borate evaporites, mineral deposits that emerge from evaporating water, are favoured by the presumptive occurrence of evaporitic basins, whether continental or marine on a prebiotic Earth (as discussed in Anovitz and Grew 2002, and in Grew et al. 2011). On the other the concentration of formamide, which is characterized by a boiling point of 210°C in the absence of eutectic with water. In this scenario, both the formation of ribose catalyzed by borates and polymerization processes of nucleotides in formamide as thermodynamically favourable environment, are hypothesized (Benner et al. 2006). Based on our previous results on formamide chemistry, recently reviewed (Saladino et al. 2007), we reasoned that formamide could also oligomerize in the presence of mineral borates. No previous studies are available on the role of borates in the prebiotic synthesis of biomolecules other than sugars. We report that borates, whose structures encompass a large variety of elemental compositions and chemical properties, catalyze the synthesis of nucleic acid bases, amino acids and biogenic carboxylic acids from formamide. The yield and selectivity of these reactions was found to depend on the mineral used in the condensation. These data, in connection with the recent use of H3BO3 as a catalyst for the polymerization of amino acids (Kolitz et al. 2009), further support a major role of borate minerals in prebiotic processes.
Material and Methods
Formamide (Fluka, >99%) was used without further purification. Gas-chromatography mass-spectrometry (GC-MS) analyses were performed with a HP5890II gas chromatograph and a Shimadzu GC-MS QP5050A with a Variant CP8944 column (WCOT fused silica, film thickness 0.25 μm, stationary phase VF-5 ms, Øί 0.25 mm, lenght 30 m). Samples were analyzed after treatment with N,N-bis-trimethylsilylacetamide in pyridine using standard procedures and betulin as internal standard for quantitative analyses. When necessary, purifications were performed on chromatography columns packed with Merck silica gel, 230–400 mesh for flash technique. Nuclear Magnetic Resonance spectra (1H-NMR and 13C-NMR) were recorded on a Bruker (200 MHz) spectrometer and are reported in δ (ppm) value. The borate minerals were obtained from Ezio Curti (ezio.curti@gmail.it), former provider and consultant of the collection of the minerals of the Department of Mineralogy (University of Rome “Sapienza”, Italy).
The provenance of the minerals is: axinite-(Mn), Harz Mountain, Germany; borax, Sigma-Aldrich; canavesite, Brosso Mine, Canavese, Piedmont, Italy; chambersite, Venice Salt Dome, Plaque Mine Paris, Louisiana USA; colemanite, Furnace Creek, Death Valley, California USA; dravite, Drava River, Austria; dumortierite, Soavina Mine, Madagascar; elbaite, Jonas, Minas Gerais, Brazil; hydroboracite, Boron, Kern County, California USA; kernite, Boron, Kern County, California USA; kornerupine, Itrongay, Madagascar; kurnakovite, Boron, Kern County, California USA; ludwigite, Corcolle querry, Rome, Latium, Italy; painite, Ohngaing, Myanmar; rhodizite, Manandona Valley, Madagascar; schorl, Galileia, Minas Gerais, Brazil; ulexite, Boron, Kramer District, Kern County, California USA; vonsenite, Vetralla, Latium Viterbo, Italy.
Reactions were performed with homogenous material isolated under the microscope, washed twice (with ethanol and analytic grade distilled water), air dried, then manually ground in a ceramic mortar. Isolation of homogeneous material under the microscope was performed mechanically, selecting the fragments of the wanted mineral resulting from a previously performed crushing procedure, discarding the spurious components. Samples of crushed materials are available upon request, except for canavesite, chambersite and kornerupine, which may be purchased at Dakota Matrix Minerals (www.dakotamatrix.com) and John Betts Fine Minerals (www.johnbetts-fineminerals.com).
Formamide Condensation. General Procedure
Formamide 1 (5.7 g, 5 mL, 0.12 mmol) was heated at 160°C for 48 h in the presence of the appropriate borate mineral (2.0% in weight). At the end of the reaction the mixture was cooled and the residual mineral was recovered by centrifugation with a Heraeus Biofuge 15 apparatus, washed with a little amount of neat formamide (0.5 ml) and separated again by centrifugation. The organic phase was evaporated under high vacuum to yield a dark crude that was analyzed by GC-MS analyses. Selected mass spectrometric data of compounds 2–17 are reported in Table 1. In the case of kernite and colemanite, which are characterized by a high solubility in formamide, the GC-MS analysis was performed directly on the crude of the reaction.
In the case of axinite-(Mn) the reaction was repeated in higher amount and the crude, after purification (flash-chromatography), was analyzed by NMR techniques.
Selected spectroscopic data:
-
Uracil (4): m.p. >300°C; 1H NMR (DMSO-d6) δ ppm: 11.0 (3H, s, NH, NH2), 7.38 (1H, m, CH), 5.50 (1H, m, CH). 13C NMR (DMSO-d6) δ ppm: 164.10 (C = O), 153.10 (C = O), 142.10 (2xCH).
-
Isocytosine (5): m.p. 275°C; 1H NMR (DMSO-d6) δ ppm: 7.42 (3H, s, NH, NH2), 7.20 (1H, d, J = 7.43 MHz, CH), 5.62 (1H, d, J = 7.43 MHz, CH). 13C NMR (DMSO-d6) δ ppm: 171.07 (C = O), 150.06 (C), 131.79 (CH), 110.2 (CH).
-
Cytosine (6): m.p. >300°C; 1H NMR (DMSO-d6) δ ppm: 7.30 (1H, m, CH), 7.10 (3H, s, NH, NH2), 5.70 (1H, m, CH). 13C NMR (DMSO-d6) δ ppm: 168.10 (C = O), 158.20 (C), 142.11 (CH), 93.10 (CH).
-
Purine (7): m.p. 214–217°C; 1H NMR (DMSO-d6) δ ppm: 9.12 (1H, s, CH), 8.90(1H, s, CH), 8.61 (1H, s, CH). 13C NMR (DMSO-d6) δ ppm: 154.66 (C), 152.0 (C), 146.10 (CH), 145.51 (CH), 130.34 (C).
-
Adenine (8): m.p. >360°C; 1H NMR (D2O+DCl) δ ppm: 8.60 (1H, s, CH), 8.50 (1H, s, CH). 13C NMR (D2O+DCl) δ ppm: 152.71 (C), 150.96 (C), 147.43 (CH), 145.76 (CH), 121.70 (C).
-
Lactic acid (10):): m.p. 52–45°C; 1H NMR (CDCl3) δ ppm: 4.31 (1H, m, CH), 1.38 (3H, m, CH3) . 13C NMR (CDCl3) δ ppm: 178.10 (C = O), 62.12 (CH), 68.49 (CH), 20.0 (CH3).
-
Lactic acid dimer (11): oil; 1H NMR (CDCl3) δ ppm: 5.11 (1H, m, CH), 4.49 (1H, m, CH), 1.58 (3H, m, CH3), 1.40 (3H, m, CH3) . 13C NMR (CDCl3) δ ppm: 175.20 (C = O), 171.91 (C = O), 72.43 (CH), 68.49 (CH), 20.13 (CH3), 18.40 (CH3).
-
Oxalic acid (12): m.p. 189.5°C; 13C NMR (D2O) δ ppm: 161.80 (C = O).
-
Pyruvic acid (14): oil; 1H NMR (CDCl3) δ ppm: 8.70 (1H, br. s, OH), 2.51 (3H, s, CH3) . 13C NMR (CDCl3) δ ppm: 194.10 (C = O), 161.80 (C = O), 26.13 (CH3).
-
Glyoxylic acid (15): m.p. 49–52°C; 1H NMR (CDCl3) δ ppm: 9.66 (1H, br. s, OH), 9.42 (1H, s, CH) . 13C NMR (CDCl3) δ ppm: 192.57 (HCO), 165.55 (COOH).
-
N-formylglycine (16): m.p. 149–151°C; 1H NMR (DMSO-d6) δ ppm: 8.22 (1H, br. s, OH), 8.06 (1H, s, CHO), 3.86-3.78 (2H, m, CH2). 13C NMR (DMSO-d6) δppm: 174.62 (C), 164.33 (C), 41.53 (CH2).
Results and Discussion
Heat-driven formamide condensation experiments were performed in the presence of a large panel of borate minerals whose elemental composition is reported in Table 2. These minerals are arranged in different groups on the basis of the Dana’s classification rules depending on the type of boron-oxygen radical (e.g., the degree of association of elementary structural units such as B-triangles and B-tetrahedra) and on their chemical and physical properties (Gaines et al. 1997). The following selected Dana’s groups were: a) Hydrates of borates containing hydroxyl or halogen moieties (group A), borax (sodium tetraborate), colemanite, hydroboracite, kernite, kurnakovite and ulexite; b) Anhydrous borates containing hydroxyl or halogen moieties (group B), chambersite, hambergite, ludwigite, rhodizite and vonsenite; c) Borosilicates (group C), dravite, dumortierite, elbaite, kornerupine, axinite-(Mn) and schorl; d) Borocarbonates (group D) represented by canavesite; e) Multiple oxides (group E) represented by painite. Synthetic boron derivatives boron anhydride and sodium perborate were also used for comparison. Borax, a mineral occurring commonly on extant Earth, abundant in evaporates in desert environments, is particularly relevant in a prebiotic perspective. An alternative system for grouping the boron minerals has also been proposed (Hawthorne et al. 2002), organizing them according to the hierarchical organization based on fundamental building blocks of the structures. We will not go into the differences in these classification systems here. We analyze a large panel of boron minerals and refrain from indicating which (or which sub-class of) boron minerals might have played the most relevant role in prebiotic chemistry. Important discussion on abundance, occurrence, origin and age of these minerals are in Dunn (1995), Ciriotti et al. (2009), Grew and Hazen (2010), Grew et al. (2011).
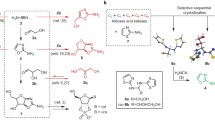
As a general procedure, the syntheses were performed by heating pure formamide 1 (5 ml; 0.126 mol) at 160°C for 48 h in the presence of catalytic amounts of the appropriate mineral (2% in weight). Given the uncertainty of the stoichiometry of the reaction, the yield was reported as mg of product formed per gram of formamide. We focused on the characterization of the more abundant products by gas chromatography/mass spectrometry analysis (GC-MS analysis) by comparison with authentic samples and, in a selected case [axinite-(Mn)], by Nuclear Magnetic Resonance analysis (1H-NMR and 13C-NMR) on purified sample. The condensation of 1 in the absence of borate minerals afforded purine as the only recovered product (c.a. 34.0 mg per gram of formamide). In the presence of borate minerals a large panel of compounds relevant for the origin of the genetic and metabolic apparatuses was obtained including components of nucleic acids and heterocycle derivatives 2–8 (Scheme 1, Table 3), carbodiimide 9, carboxylic acids 10–15 and amino acid derivatives 16–17 (Scheme 2, Table 4).
Synthesis of Nucleic Acid and Heterocycle Derivatives
The synthesis of nucleobases from formamide requires the generation “in situ” of HCN and of others low molecular weight compounds, such as formaldehyde and ammonium formate, that are involved in the condensation process (Saladino et al. 2005b). Two main reaction pathways are suggested for the condensation. Yamada and Okamoto (1972) described the formation of a substituted 5,6-dihydro pyrimidinone derivative as a common intermediate for nucleobases, produced by a sequential condensation of formamide and HCN. In this latter case, the reduction of two exocyclic imino moieties on the pyrimidine ring was a specific redox step to obtain the C-5/C-6 double bond in pyrimidine nucleobases. As an alternative, purines can be synthesized by a multi-steps process involving HCN oligomers, 5-imidazole carbonitrile and 5-imidazole carboxyamide (not shown), that are also key intermediate in the polymerization of HCN (Sanchez et al. 1966; Voet and Schwartz 1983; Schwartz and Bakker 1989; Saladino et al. 2007).
Mineral borates catalyzed the synthesis of both purine and pyrimidine nucleobases. Uracil 4, cytosine 6 and adenine 8 are DNA and RNA nucleobases, while isocytosine [2-aminopyrimidinin-4(3H)-one, iC] 5, that is not a component of nucleic acids, recognizes 6 through a Watson-Crick hydrogen bonding pattern similar to that of the guanine/cytosine pair (Zhanpeisov and Leszczynski 1999). Compound 5 also recognizes guanine by a reversed Watson-Crick interaction (Gupta et al. 2004). 2(1H)-pyrimidinone 3 was previously synthesized from formamide in the presence of iron sulphur and iron-copper sulphur minerals (Saladino et al. 2008), such as pyrite (FeS2) and pyrrhotite (Fe(1-x)S), key reagents in the Wächtershäuser model of the chemoautotrophic origin of the secondary metabolism (Wächtershäuser 1988; Wächtershäuser 1990; Wächtershäuser 1992). Compound 3 is an alternative to natural pyrimidine nucleobases in a pre-RNA world due to reported synthesis of the corresponding pyrimidinone ribonucleoside in a plausible prebiotic scenario (Bean et al. 2007). The 3-hydroxy pyrimidine 2 was not previously detected in formamide-based syntheses, while the 4(3H)-pyrimidinone (4-hydroxy pyrimidine in the tautomeric form), a minor component in the Murchison meteorite, was obtained from formamide in the presence of cosmic dust analogues of terrestrial olivines (Saladino et al. 2005a).
Irrespective of the nature of the borate mineral, purine 7 was recovered as the main reaction product. Adenine 8 was synthesized in the presence of anhydrous borates (Table 3, entries 7–11) and of painite (Table 3, entry 19), but not in the presence of hydrates of borates and canavesite. The borosilicates showed an intermediate behaviour, as in the case of manganaxinite and dravite (Table 3, entries 16 and 12). Note that 8 was not obtained with the simple boron compounds used as references (Table 3, entries 20–21). Since the synthesis of 8 from formamide invariably requires the generation of hydrogen cyanide (HCN) (Yamada et al. 1978), it is reasonable to suggest that hydrates of borates, borocarbonates and simple boron derivatives were not efficient catalysts for this transformation. The generation of HCN during the condensation of formamide was confirmed by the detection of the HCN-tetramer, diaminomaleodinitrile (DAMN), in the presence of dravite and vonsenite (note [l], Table 3) (Orgel 2004). The selectivity in the synthesis of compounds 5–6 requires the following discussion. Compound 5 was produced by a number of borate minerals higher than that available for 6, hydrates of borates (Table 3, entries 3 and 5), anhydrous borates (Table 3, entries 7 and 9–10), borocarbonate (Table 3, entry 18) and borosilicates (Table 3, entry 12) being the best catalysts. The reaction pathway for the formation of 5 from formamide is similar to that for 6 (Yamada et al. 1978), the only difference being the selectivity in the addition/elimination process of ammonia as nucleophile on the substituted 5,6-dihydro pyrimidinone intermediate (that is C-2 versus C-4 addition/elimination process. For the numbering of the pyrimidinone ring see Scheme 1). The prevalence of 5 with mineral borates highlights the role of the mineral surface in the regioselectivity of the condensation (Botta et al. 1997). Uracil, a product of elimination of ammonia from both 5 and 6, was detectable with borosilicates (Table 3, entries 12–17), multiple oxide (Table 3, entry 19), colemanite, chambersite and vonsenite (Table 3, entries 2, 7 and 11). Hydroxypyrimidines 2–3 were obtained under various experimental conditions, dravite being the best catalyst (Table 3, entry 12).
Qualitative structure activity relationships (SAR) for each Dana’s group were also defined applying the Nickel-Strunz borate minerals classification rules (Strunz and Nickel 2001). As examples, in the borosilicates, the member of the [Si6O18]12− six membered single ring sub-class, dravite was more reactive than sorosilicates (kornerupine) and neosilicates with BO3 triangles and B[4] and Be[4] tetrahedra [durmortierite and axinite-(Mn)]. Similarly, the hydrates of borates classified as triborates (colemanite and kurnakovite) were more reactive than tetraborates and pentaborates. Monoborates were the more reactive anhydrous borates.
Synthesis of Biogenic Carboxylic Acids and Amino Acid Derivatives
In addition to compounds 2–8, carbodiimide, carboxylic acids and amino acid derivatives were synthesized (compounds 9–17, Scheme 2 and Table 4). Biogenic carboxylic acids lactic acid 10 and pyruvic acid 14 are intermediates in the reductive version of the citric acid cycle. This cycle was suggested by Smith and Morowitz (2004) as a primordial autocatalytic reaction pathway for the reductive carbon assimilation of carbon oxides in a primordial metabolism (Morowitz et al. 2000). The variant of the conventional (oxidative) citric acid cycle is known to be a metabolic process in some bacteria (Holms 1987). Examples of prebiotic synthesis of intermediates of the reductive version of the citric acid cycle were previously reported for the formation in small yield of pyruvic acid 14 from carbon monoxide (CO) and iron sulfide and alkanethiols (Cody et al. 2000), and the formation of acetic acid form CO in with iron-nickel and sulfides (Huber and Wächtershäuser 1997). Moreover, traces of ketoglutaric acid were obtained from succinate in the presence of zinc sulfide under photochemical conditions (Zhang and Martin 2006). Glycolic acid 13 is involved in the photosynthetic oxidation of glycolate to glyoxylate (Smith et al. 1997), while glyoxylic acid 15 is an intermediate (in the salt form) in the glyoxylate cycle in bacteria, fungi and plants (Escher and Widmer 1997). Compounds 10 and 13–15 can be produced, in accordance to Eschenmoser suggestions (Eschenmoser 2007), through a common reaction pathway involving a reactive HCN-dimer and/or DAMN. The hydrolysis of the nitrile moiety in DAMN affords hydroxyoxaloacetic acid tautomers (2,3-dihydroxymaleic, 2,3-dihydroxyfumaric, and 2-hydroxyoxaloacetic acid, respectively) that are easily converted to oxaloacetic acid (not shown) and its decarboxylation product 14 via a stepwise addition of over two electrons and two protons (Eschenmoser and Loewenthal 1992; Koerber et al. 2005). Further reduction of 14 yields 10. In a similar way, 15 can be formed by hydrolysis of the HCN-dimer, and 13 by a second two electrons-two protons reductive step of the formyl moiety in 15.
The presence of the dimer of lactic acid, compound 11, is also of interest. In fact, even though 11 is not an intermediate of present day metabolism, its formation suggests the possibility of spontaneous dimerization processes during the condensation of formamide, possibly catalyzed by carbodiimide 9. Compound 9 is a well-known condensing agent for the synthesis of peptides and oligonucleotides starting from deactivated monomers and, in principle, it may play a role in abiotic polymerization processes (Slebocka-Tilk et al. 2002). As previously reported, 9 was produced by elimination of H2O from the urea generated in situ during formamide condensation (Costanzo et al. 2007). This hypothesis was confirmed by the efficient synthesis of 11 treating 10 in the presence of 1-ethyl-3(3-dimethylamino)carbodiimide (EDC), a commercially available and relatively stable derivative of carbodiimide. The synthesis of N-formylglycine 16 was previously reported in the presence of mineral phosphates to occur by a Strecker condensation between HCN, formaldehyde and ammonia (Miller 1953), followed by a N-formylation step with excess formamide (Aizpurua and Palomo 1983). N-formyl alanine 17 was not previously obtained from formamide. In this latter case, a Strecker condensation involving acetamide can be suggested, even though the methylation of 16 with formaldehyde, or the amination of pyruvic acid with ammonia, cannot be completely ruled out. An example of the alkylating properties of formaldehyde in the presence of minerals was reported by Kulkarni et al. (2008). The formation of amino acids from their ketone precursors by reductive amination or transamination are well documented reactions (Doctor and Orò 1967; Huber and Wächtershäuser 2003). Moreover, hydrolysis of DAMN under vigorous conditions has been reported to afford mainly glycine in the presence of small amounts of alanine and aspartic acid (Sanchez et al. 1967; Ferris et al. 1978).
As for the selectivity of the reactions, all mineral borates behaved as catalysts for the synthesis of compounds 10–12. A major selectivity was observed for carboxylic acids 13–15, as exemplified by pyruvic acid 14 that was synthesized only in the presence of borosilicates (Table 4, entries 12–17) and painite (Table 4, entry 19). Borosilicates were also the best catalysts for the preparation of glyoxylic acid 15. Hydrates of borates (with the only exception of borax and kernite) (Table 4, entries 1–6) and one of the anhydrous borate group, chambersite (Table 4, entry 7), were also catalysts for the synthesis of both N-formylglycine 16 and N-formyl alanine 17. Several borosilicates (kornerupine, axinite-(Mn) and schorl) (Table 4, entries 15–17) and one mineral of the anhydrous borate group (vonsenite) (Table 4, entry 11) selectively catalyzed the synthesis of 16, while 17 was detected in the presence of ludwigite, elbaite and painite (Table 4, entries 9, 14 and 19).
Conclusions
In the context of the origin of nucleic acids, dry-wet cycles were suggested as possible mechanisms for the solution of the “water paradox”, the thermodynamic conundrum impairing the likelihood of polymerizations to occur and give rise to stable polymers. Water is the only environment in which RNA and DNA conceivably evolved to their-present function and its chemistry has necessarily entered the prebiotic scenario at some point during the evolution of chemical pre-genetic complexity. In addition, dry-wet cycles also offer plausible frames for the prebiotic chemistry of formamide and mineral borates. Formamide, the product of hydrolysis of HCN, is easily concentrated by evaporation of water, thus accumulating in dry environments. The possible hydrolysis of formamide to ammonium formate is not deleterious in this context, being ammonium formate a known precursor for the synthesis of purine nucleobases (Hill and Orgel 2002). Borate minerals are often found on Earth as evaporites, the mineral deposits produced during the evaporation of water. Thus, in principle, it appears that in a coherent set of conditions, essentially consisting of alternating wet-dry cycles, the possible concentration of formamide and the formation of deposits of boron minerals in the form of evaporites (Grew et al. 2011), were all contemporaneously possible. Hence, the relevance of the question of whether formamide and mineral borates are effective reagents in the processes leading to the synthesis of compounds necessary for the origin of life. The data reported show that formamide oligomerizes in the presence of borate minerals yielding, at the same time, nucleobases and biogenic carboxylic acids. The synthesis of these compounds entails common precursors as HCN-oligomers. Noteworthy, different mineral studied were able to catalyze the contemporary synthesis of both classes of biomolecules. The very fact that a one-pot, one-catalyst reaction affords the building blocks of both genetic and metabolic apparatuses highlights the interest of formamide-borates chemistry. The presence of amino acid derivatives, and the known catalytic effects of borates on prebiotic synthesis of ribose, further increase the interest of this experimental model.
References
Aizpurua JM, Palomo C (1983) Reagents and synthetic methods 30. Practical and improved method for formylating amino compounds by means of formic acid-dimethylformamide. System. Synth Commun 13:745–752
Anovitz LM, Grew ES (2002) Mineralogy, petrology and geochemistry of boron: an introduction. In: Grew ES, Anovitz LM (eds), Boron: mineralogy, petrology and geochemistry. Rev Mineral 33, 2nd printing. Mineralogical Society of America, Washington, pp 1–40, 115–116, 38
Bean HD, Sheng Y, Collins JP, Anet JFAL, Leszczynski FJ, Hud NV (2007) Formation of a β-pyrimidine nucleoside by a free pyrimidine base and ribose in a plausible prebiotic reaction. J Am Chem Soc 129:9556–9557
Benner SA, Carrigan MA, Ricardo A, Frye F (2006) Setting the stage: the history, chemistry, and geobiology behind RNA. In: Gesteland RF, Cech TR, Atkins JF (eds) The RNA World, 3rd edn. Cold Spring Harbor Laboratory Press, USA, pp 1–21
Botta M, Occhionero F, Saladino R, Crestini C, Nicoletti R (1997) An unexpected and efficient direct nucleophilic C-4 hydroxy substitution on 2-methoxy- and 2-methylthio-4(3H-pyrimidinones bearing a diethylamino moiety on the C-6 side chain. Tetrahedron Lett 38(47):8249–8252
Ciriotti ME, Fascio L, Pasero M (2009) Italian type minerals. Plus-Pisa University Press, Pisa
Cody GD, Boctor NZ, Filley TR, Hazen RM, Scott JH, Sharma A, Yoder HS Jr (2000) Primordial carbonylated iron-sulfur compounds and the synthesis of pyruvate. Science 289:1337–1340
Costanzo G, Saladino R, Crestini C, Ciciriello F, Di Mauro E (2007) Formamide as the main building block in the origin of nucleic acids. BMC Evol Biol 7:1471–1482
Doctor VM, Orò J (1967) Non-enzymatic transamination of histidine with α-keto acids. Naturwissenschaften 54:443
Dunn PJ (1995) Franklin and Sterling Hill, New Jersey: the world’s most magnificent mineral deposits. Part 4. Descriptive mineralogy. Phyllosilicates - layer silicates, tectosilicates, elements, sulfides, arsenides, antimonides, sulfosalts, oxides, halides, carbonates. Franklin, The Franklin-Ogdensburg Mineralogical Society
Eschenmoser A (2007) On a hypothetical generational relationship between HCN and constituents of the reductive citric acid cycle. Chem Biodivers 4:554–573
Eschenmoser A, Loewenthal E (1992) Chemistry of potentially prebiological natural products. Chem Soc Rev 21:1–16
Escher CL, Widmer F (1997) Lipid mobilization and gluconeogenesis in plants: do glyoxylate cycle enzyme activities constitute a real cycle? A hypothesis. Biol Chem 378:803–813
Ferris JP, Joshi PC, Edelson EH, Lawless JG (1978) HCN: a plausible source of purines, pyrimidines and amino acids on the primitive earth. J Mol Evol 11:293–311
Gaines RV, Skinner HCW, Foord EE, Rosenzweig A (1997) Dana’s new mineralogy (Eighth Edition), John Wiley & Sons, Inc.
Grew E, Hazen RM (2010) Evolution of boron minerals: has early species diversity been lost from the geological record? Geol Soc Am Abstr Programs 42(5):92
Grew ES, Bada JL, Hazen RM (2011) Borate minerals and origin of the RNA world. Orig Life Evol Biosph. doi:10.1007/s11084-010-9233-y
Gupta D, Huelsekopf M, Cerdà MM, Ludwig R, Lippert B (2004) Complex formation of Isocytosine Tautomers with Pd(II) and Pt(II). Inorg Chem 43:3386–3393
Hawthorne FC, Burns PC, Grice JD (2002) The crystal chemistry of boron. In: Grew ES, Anovitz LM (eds) Boron: mineralogy, petrology and geochemistry. Rev Mineral 33, 2nd printing, Mineralogical Society of America, Washington, D.C., pp. 41–116, 385
Hill A, Orgel LE (2002) Synthesis of adenine from HCN tetramer and ammonium formate. Orig Life Evol Biosph 32:99–102
Holms WH (1987) Control of flux through the citric acid cycle and the glyoxylate by-pass in Escherichia coli. Biochem Soc Symp 54:17–31
Huber C, Wächtershäuser G (1997) Activated acetic acid by carbon fixation on (Fe, Ni)S under primordial conditions. Science 276:245–247
Huber C, Wächtershäuser G (2003) Primordial reductive amination revisited. Tetrahedron Lett 44:1695–1697
Koerber K, Risch P, Bruckner R (2005) A novel strategy for the convergent synthesis of polyols: enone formation, asymmetric dihydroxylation, reductive cleavage, hydride addition. Synlett 19:2905
Kolitz M, Cohen-Arazi N, Hagag I, Katzhendler J, Domb AJ (2009) Biodegradable polyesters derived from amino acids. Macromolecules 42:4520–4530
Kulkarni SJ, Madhavi G, Ramachander Rao A, Krishna Mohan KVV (2008) Side-chain alkylation of acetophenone with formaldehyde over alkali and alkaline earth metal ion modified basic zeolites. Catalysis Commun 9:532–538
Miller SL (1953) Production of amino acids under possible primitive earth conditions. Science 117:528–529
Morowitz HJ, Kostelnik J, Yang J, Cody GD (2000) The origin of intermediary metabolism. Proc Natl Acad Sci, USA 97:77047707
Orgel LE (2004) Prebiotic adenine revisited: eutectics and photochemistry. Orig Life Evol Biosph 34:361–369
Prieur BF (2001) Etude de l’activité prébiotique potentielle de l’acide borique. CR Acad Sci, Chim/Chem 4:667–670
Ricardo A, Carrigan MA, Olcott AN, Benner SA (2004) Borate minerals stabilize ribose. Science 303:196
Saladino R, Crestini C, Ciciriello F, Costanzo G, Negri R, Di Mauro E (2004) A novel synthesis of biomolecular precursors. Astrobiology: Future Perspective. Ehrenfreund P (ed) Netherlands, pp 393–413
Saladino R, Crestini C, Neri V, Brucato JR, Colangeli L, Ciciriello F, Di Mauro E, Costanzo G (2005a) Synthesis and degradation of nucleic acid components by formamide and cosmic dust analogues. Chembiochem 6:1368–1374
Saladino R, Crestini C, Costanzo G, Di Mauro E (2005b) On the prebiotic synthesis of nucleobases, nucleotides, oligonucleotides, pre-RNA and pre-DNA molecules. Top Curr Chem 259:29–68
Saladino R, Crestini C, Ciciriello F, Costanzo G, Di Mauro E (2006) About a formamide-based origin of informational polymers: syntheses of nucleobases and favourable thermodynamic niches for early polymers. Orig Life Evol Biosph 36:523–531
Saladino R, Crestini C, Ciciriello F, Costanzo G, Di Mauro E (2007) Formamide chemistry and the origin of informational polymers. Chem Biodivers 4:694–720
Saladino R, Crestini C, Neri V, Costanzo G, Graciotti M, Di Mauro E (2008) Synthesis and Degradation of Nucleic Acid Components by Formamide and Iron Sulfur Minerals. J Am Chem Soc 130:15512–15518
Saladino R, Crestini C, Ciciriello F, Pino S, Costanzo G, Di Mauro E (2009) From formamide to RNA: the roles of formamide and water in the evolution of chemical information. Res Microbiol 160:441–448
Sanchez RA, Ferris JP, Orgel LE (1966) Conditions for purine synthesis: did prebiotic synthesis occur at low temperature? Science 153:72–73
Sanchez RA, Ferris JP, Orgel LE (1967) Studies in prebiotic synthesis. II. Synthesis of purine precursors and amino acids from aqueous hydrogen cyanide. J Mol Biol 30:223–252
Schwartz AW, Bakker CG (1989) Was adenine the first purine? Science 245:1102–1104
Scorei R, Cimpoiaşu VM (2006) Boron enhances the thermostability of carbohydrates. Orig Life Evol Biosph 36:1–11
Slebocka-Tilk H, Sauriol F, Monette M, Brown RS (2002) Aspects of the hydrolysis of formamide: revisitation of the water reaction and determination of the solvent deuterium kinetic isotope effect in base. Can J Chem 80:1343–1350
Smith E, Morowitz HJ (2004) Universality in intermediary metabolism. Proc Natl Acad Sci USA 101:13168–13171
Smith AD, Datta SP, Smith GH, Campbell PN, Bentley R (1997) Oxford dictionary of biochemistry and molecular biology. Oxford University Press, New York
Strunz H, Nickel EH (2001) Mineralogical tables: chemical-structural mineral classification system. In: Stuttgart E (ed) Mineralogical Tables. Chemical Structural Mineral Classification System, Schweizerbart’sche Verlagsbuchhandlung pp 870
Voet AB, Schwartz AW (1983) Uracil synthesis via HCN oligomerization. Orig Life 12:45–49
Wächtershäuser G (1988) Before enzymes and templates: theory of surface metabolism. Syst Appl Microbiol 52:452–484
Wächtershäuser G (1990) Evolution of the first metabolic cycles. Proc Natl Acad Sci USA 87:200–203
Wächtershäuser G (1992) Groundworks for an evolutionary biochemistry: the iron-sulphur world. Prog Biophys Mol Biol 58:85–201
Yamada H, Okamoto T (1972) A one-step synthesis of purine ring from formamide. Chem Pharm Bull 20:623
Yamada H, Hirobe M, Higashiyama K, Takahashi H, Suzuki KT (1978) Reaction mechanism for purine ring formation as studied by 13C-15N coupling. Tetrahedron Lett 42:4039–4042
Zhang XV, Martin ST (2006) Driving parts of krebs cycle in reverse though mineral photochemistry. J Am Chem Soc 128:16032–16033
Zhanpeisov NU, Leszczynski J (1999) Specific solvation effects on the structures and properties of Watson–Crick and reverse Watson–Crick isocytosine–cytosine and guanine–cytosine base pairs: a theoretical ab initio study. J Mol Struct THEOCHEM 487:107–115
Acknowledgement
UE Cost Action CMO703 System Chemistry and the project ASI/INAF/015/07/0 are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saladino, R., Barontini, M., Cossetti, C. et al. The Effects of Borate Minerals on the Synthesis of Nucleic Acid Bases, Amino Acids and Biogenic Carboxylic Acids from Formamide. Orig Life Evol Biosph 41, 317–330 (2011). https://doi.org/10.1007/s11084-011-9236-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-011-9236-3