Abstract
Purpose
Canal dredging and shaping produce considerable amounts of sediments whose reuse on- and off-site depends on their pollution level. This study explores the potential of a calcium chloride washing to remove potentially toxic elements (PTEs) from a freshly dredged calcareous sediment and to affect the aggregate stability of the washed sediment.
Materials and methods
The ability of 0.5-M CaCl2 washing to restore the sediment samples polluted with Cu and Zn dredged from a freshwater canal was assessed. The distribution of the two metals among the sediment geochemical phases, the potential availability to plants (DTPA), and the water aggregate stability of particles were evaluated on the washed samples and compared to control.
Results and discussion
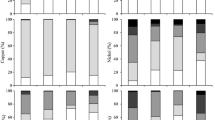
In the most polluted sample (~200 and 500 mg kg−1 of Cu and Zn, respectively), the washing decreased the amount of Cu by 26%, mainly in the sulfide/organic fraction, and of Zn by 10%, mainly in carbonates. A decrease in the dispersivity of clay fraction was observed due to the well-known effect of Ca2+ ions on flocculation of colloidal clay particles. At large scale, the aggregates formed by the interaction between large particles and flocculated clay showed lower water stability with respect to the control, thus suggesting the need to improve the physical properties of the treated material. Noteworthy, after 1 year of dry storage from washing, the average percentage of potentially bioavailable (DTPA) Cu and Zn (23 and 13% of pseudo-total amount, respectively) dropped with respect to the control (40 and 19%) and a concomitant carbonate increase (+33%) was observed.
Conclusions
The CaCl2 washing is a promising method to reduce Cu and, to a minor extent, Zn in wet calcareous sediments dredged from freshwater canals. However, there is still room for improvement. The PTE fractions remaining in carbonates and organic component of washed sediment clearly indicate the potential of combining mild acidic pH values with calcium chloride in the washing to enhance the PTE removal.

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Ahmad A (2009) Study on effect of HCl and CaCl2 on extraction of heavy metals from contaminated soils. Asian J Chem 21:1690–1698
Argese E, Bettiol C, Cedolin A, Bertini S, Delaney E (2003) Speciation of heavy metals in sediments of the lagoon of Venice collected in the industrial area. Ann Chim 93:329–336
Attou F, Bruand A, Le Bissonnais Y (1998) Effect of clay content and silt-clay fabric on stability of artificial aggregates. Eur J Soil Sci 49:569–577
Bronick CJ, Lal R (2005) Soil structure and management: a review. Geoderma 124:3–22
Carlon C (ed) (2007) Derivation methods of soil screening values in Europe. A review and evaluation of national procedures towards harmonization. European Commission, Joint Research Centre, Ispra, EUR 22805-EN, 306
Chesworth W (2008) Carbonates. In: Chesworth W (ed) Encyclopedia of soil science. Springer Netherlands, Dordrecht, pp 99–101
Chesworth W, Camps Arbestain M, Macías F (2008) Calcareous soils. In: Chesworth W (ed) Encyclopedia of soil science. Springer Netherlands, Dordrecht, pp 77–79
Dal Ferro N, Berti A, Francioso O, Ferrari E, Matthews GP, Morari F (2012) Investigating the effects of wettability and pore size distribution on aggregate stability: the role of soil organic matter and the humic fraction. Eur J Soil Sci 63:152–164
Dermont G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152:1–31
Directive 2000/60/CE (2000) http://data.europa.eu/eli/dir/2000/60/oj
Directive 2013/39/UE (2013) http://data.europa.eu/eli/dir/2013/39/oj. Accessed 24 Aug 2013
Falsone G, Celi L, Bonifacio E (2007) Aggregate formation in chloritic and serpentinitic alpine soils. Soil Sci 172:1019–1030
Ferronato C, Vianello G, Vittori Antisari L (2015) Heavy metal risk assessment after oxidation of dredged sediments through speciation and availability studies in the Reno river basin, northern Italy. J Soils Sediments 15:1235–1245
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis. Part I. ASA and SSSA, Madison, WI, USA, pp 383–411
Hansen DJ, Berry WJ, Boothman WS, Pesch CE, Mahony JD, Di Toro DM, Robson DL, Ankley GT, Ma D, Yan Q (1996) Predicting the toxicity of metal-contaminated field sediments using interstitial concentration of metals and acid-volatile sulfide normalizations. Environ Toxicol Chem 15:2080–2094
Hooda PS (2010) Trace elements in soils. Kingston University London, UK
Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Revi Environ Sci Bio 9:215–288
Kelderman P, Osman AA (2007) Effect of redox potential on heavy metal binding forms in polluted canal sediments in Delft (the Netherlands). Water Res 41:4251–4261
Kemper WD, Rosenau RC (1986) Aggregate stability and size distribution. In: Klute A (ed) Methods of soil analysis. Part I. ASA and SSSA, Madison, WI, USA, pp 425–442
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manag 28:215–225
Le Bissonnais Y, Arrouays D (1997) Aggregate stability and assessment of soil crustability and erodibility: II. Application to humic loamy soils with various organic carbon contents. Eur J Soil Sci 48:39–48
Legislative Decree 152/2006 (2006) G.U.- Gazzetta Ufficiale delle Repubblica Italiana, Norme in materia ambientale, D.L. 152/2006. n.88
Li YJ, Hu PJ, Zhao J, Dong CX (2015) Remediation of cadmium- and lead-contaminated agricultural soil by composite washing with chlorides and citric acid. Environ Sci Pollut Res 22:5563–5571
Liang C, Peng X (2017) Mobilization of arsenic from contaminated sediment by anionic and nonionic surfactants. J Environ Sci 56:281–289
Madrid L, Diaz-Barrientos E, Ruiz-Cortes E, Reinoso R, Biasioli M, Davidson CM, Duarte AC, Grčman H, Hossack I, Hursthouse AS, Kralj T, Ljung K, Otabbong E, Rodrigues S, Urquhart GJ, Ajmone-Marsan F (2006) Variability in concentrations of potentially toxic elements in urban parks from six European cities. J Environ Monit 8:1158–1165
Maejima Y, Makino T, Takano H, Kamiya T, Sekiya N, Itou T (2007) Remediation of cadmium-contaminated paddy soils by washing with chemicals: effect of soil washing on cadmium uptake by soybean. Chemosphere 67:748–754
Makino T, Kamiya T, Takano H, Itou T, Sekiya N, Sasaki K, Maejima Y, Sugahara K (2007) Remediation of cadmium-contaminated paddy soils by washing with calcium chloride: verification of on-site washing. Environ Pollut 147:112–119
Makino T, Sugahara K, Sakurai Y, Takano H, Kamiya T, Sasaki K, Itou T, Sekiya N (2006) Remediation of cadmium contamination in paddy soils by washing with chemicals: selection of washing chemicals. Environ Pollut 144:2–10
Motuzova GV, Makarychev IP, Dergham HM, Stepanov AA, Barsova NU (2012) Soil organic matter and their interactions with metals: processes, factors, ecological significance. Nova Science Publishers, New York
Peng JF, Song YH, Yuan P, Cui XY, Qiu GL (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640
Plant JA, Voulvoulis N, Ragnarsdottir KV (2012) Pollutants, human health and the environment: a risk based approach. Wiley-Blackwell
Procedure 2006/0086/COD. COM (2006) 232: Proposal for a Directive of the European Parliament and of the Council establishing a framework for the protection of soil and amending Directive 2004/35/EC https://eur-lex.europa.eu/procedure/EN/2006_86
Pulatsü S, Topçu A (2010) Review of 15 years of research on sediment heavy metal contents and sediment nutrient release in inland aquatic ecosystems, Turkey. J Water Res Prot 7:85–100
Rauret G, Rubio R, López-Sánchez JF, Casassas E (1988) Determination and speciation of copper and lead in sediments of a Mediterranean river (River Tenes, Catalonia, Spain). Water Res 22:449–455
Rulkens WH, Bruning H (2005) Cleanup technologies for dredged fine sediments: review and future challenges. In: Olfenbuttel RF, White PJ (eds) Remediation of Contaminated Sediments–2005: Finding Achievable Risk Reduction Solutions. Proceedings of the third international conference on Remediation of contaminated sediments (New Orleans, LA, USA, January , 2005), Battelle Press, Columbus, Ohio, US, pp C6–C01
Saeki K, Okazaki M, Matsumoto S (1993) The chemical phase changes in heavy metals with drying and oxidation of the lake sediments. Water Res 27:1243–1251
Samourgiannidis G, Matsi T (2013) Comparison of two sequential extraction methods and the DTPA method for the extraction of micronutrients from acidic soils. Commun Soil Sci Plant Anal 44:38–49
Sanz LH (2011) Heavy metal sediments. Nova Science Publishers Inc, New York
Sastre-Conde I, Lobo MC, Beltrán-Hernández RI, Poggi-Varaldo HM (2015) Remediation of saline soils by a two-step process: washing and amendment with sludge. Geoderma 247-248:140–150
Sepahvand H, Forghani A (2012) Comparison of two sequential extraction procedures for the fractionation of zinc in agricultural calcareous soils. Chem Speciat Bioavailab 24:13–22
Soil Survey Division Staff (1993) Soil survey manual. U.S. Department of Agriculture, Soil Conservation Service
Sposito G (1989) The chemistry of soils. Oxford University Press, New York
Steinberg RV (2011) Contaminated soils: environmental impact, disposal and treatment. Nova Science Publishers, Inc, New York
Tack FM, Vandecasteele B (2008) Cycling and ecosystem impact of metals in contaminated calcareous dredged sediment-derived soils (Flanders, Belgium). Sci Total Environ 400:283–289
The heavy metal background maps of the Emilia-Romagna plain ( http://ambiente.regione.emilia-romagna.it/en/geologia/temi/heavy-metals-in-soil/heavy_metal_background_maps )
van Reeuwijk LP (2002) Procedure for soil analysis, 6th edn., ISRIC, Wageningen, Technical paper 9. FAO, pp 1–81
Violante A, Huang PM, Gadd GM (2007) Biophysico-chemical processes of heavy metals and metalloids in soil environments. John Wiley & Sons
Wang H, Liu T, Tsang DCW, Feng S (2017) Transformation of heavy metal fraction distribution in contaminated river sediment treated by chemical-enhanced washing. J Soils Sediments 17:1208–1218
Zanini E, Bonifacio E, Albertson JD, Nielsen DR (1998) Topsoil aggregate breakdown under water-saturated conditions. Soil Sci 163:288–298
Zhang L, Lu X, Liu X, Yang K, Zhou H (2016) Surface wettability of basal surfaces of clay minerals: insights from molecular dynamics simulation. Energ Fuels 30:149–160
Zoumis T, Schmidt A, Grigorova L, Calmano W (2001) Contaminants in sediments: remobilisation and demobilisation. Sci Total Environ 266:195–202
Acknowledgments
Carla Zampighi and Cinalberto Bertozzi (Burana Land Reclamation Consortium, Italy) are acknowledged for supplying the sediment samples and Franco Zinoni and Barbara Villani (ARPAE-Emilia_Romagna, Italy) for the fruitful discussion. Geo Paul is acknowledged for proofreading.
Funding
This study was supported by the University of Bologna (Grant RFO2015_2016_Braschi_Ilaria).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jos Brils
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Buscaroli, E., Sciubba, L., Falsone, G. et al. Calcium chloride washing of calcareous sediment from a freshwater canal: effect on the removal of potentially toxic elements and water aggregate stability. J Soils Sediments 19, 3098–3107 (2019). https://doi.org/10.1007/s11368-019-02298-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02298-3
Keywords
Profiles
- Enrico Buscaroli View author profile