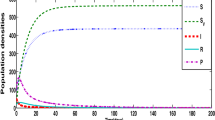
Abstract
The transmission of waterborne pathogens is a complex process that is heavily linked to the spatial characteristics of the underlying environmental matrix as well as to the temporal variability of the relevant hydroclimatological drivers. In this work, we propose a time-varying, spatially explicit network model for the dynamics of waterborne diseases. Applying Floquet theory, which allows to extend results of local stability analysis to periodic dynamical systems, we find conditions for pathogen invasion and establishment in systems characterized by fluctuating environmental forcing, thus extending to time-varying contexts the generalized reproduction numbers recently obtained for spatially explicit epidemiology of waterborne disease. We show that temporal variability may have multifaceted effects on the invasion threshold, as it can either favor pathogen invasion or make it less likely. Moreover, environmental fluctuations characterized by distinctive geographical signatures can produce diversified, highly nontrivial effects on pathogen invasion. Our study is complemented by numerical simulations, which show that pathogen establishment is neither necessary nor sufficient for large epidemic outbreaks to occur in time-varying environments. Finally, we show that our framework can be used to reliably characterize the early geography of epidemic outbreaks triggered by fluctuating environmental conditions.







Similar content being viewed by others
References
Akanda A S, Jutla S, Islam S (2009) Dual peak cholera transmission in Bengal Delta: a hydroclimatological explanation. Geophys Res Lett 36:L19401
Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P (2006) Seasonality and the dynamics of infectious diseases. Ecol Lett 9:467–484
Bacaër N (2007) Approximation of the basic reproduction number R 0 for vector-borne diseases with a periodic vector population. Bull Math Biol 69: 1067–1091
Bacaër N, Ait Dads E (2012) On the biological interpretation of a definition for the parameter R 0 in periodic population models. J Math Biol 65:601–621
Bacaër N, Gomes M G M (2009) On the final size of epidemics with seasonality. Bull Math Biol 71:1954–1966
Bacaër N, Guernaoui S (2006) The epidemic threshold of vector-borne diseases with seasonality. J Math Biol 53:421–436
Bani-Yaghoub M, Gautam R, Shuai Z, van den Driessche P (2012) Reproduction numbers for infections with free-living pathogens growing in the environment. J Biol Dyn 6:923–940
Bertuzzo E, Maritan A, Gatto M, Rodriguez-Iturbe I, Rinaldo A (2007) River networks and ecological corridors: reactive transport on fractals, migration fronts, hydrochory. Water Resour Res 43:W04419
Bertuzzo E, Gatto M, Maritan A, Azaele S, Rodriguez-Iturbe I, Rinaldo A (2008) On the space-time evolution of a cholera epidemic. Water Resour Res 44:W01424
Bertuzzo E, Casagrandi R, Gatto M, Rodriguez-Iturbe I, Rinaldo A (2010) On spatially explicit models of cholera epidemics. J R Soc Interface 7:321–333
Bertuzzo E, Mari L, Righetto L, Gatto M, Casagrandi R, Rodriguez-Iturbe I, Rinaldo A (2012) Hydroclimatology of dual-peak cholera epidemics: inferences from a spatially explicit model. Geophys Res Lett 39:L05403
Bittanti S, Colaneri P (2009) Periodic systems, filtering and control. Springer, London
Bouma M J, Pascual M (2001) Seasonal and interannual cycles of endemic cholera in Bengal 1891–1940 in relation to climate and geography. Hydrobiologia 460:147–156
Capasso V, Paveri-Fontana S L (1979) A mathematical model for the 1973 cholera epidemic in the European Mediterranean Region. Rev Epidemiol Santé Publique 27:121–132
Casagrandi R, Gatto M (2006) The intermediate dispersal principle in spatially explicit metapopulations. J Theor Biol 239:22–32
Codeço C T (2001) Endemic and epidemic dynamics of cholera: the role of the aquatic reservoir. BMC Infect Dis 1:1
Colwell R R (1996) Global climate and infectious disease: the cholera paradigm. Science 274:2025–2031
de Magny GC, Murtugudde R, Sapiano M RP, Nizam A, Brown CW, Busalacchi AJ, Yunus M, Mohammad Nair GB, Gil AI, Lanata CF, Calkins J, Manna B, Rajendran K, Bhattacharya MK, Huq A, Sack RB, Colwell RR (2008) Environmental signatures associated with cholera epidemics. Proc Natl Acad Sci USA 105:17676–17681
Diekmann O, Heesterbeek J A P (2000) Mathematical epidemiology of infectious diseases: model building, analysis and interpretation. Wiley, Chichester
Diekmann O, Heesterbeek J A P, Metz J A J (1990) On the definition and the computation of the basic reproduction ratio R 0 in models for infectious diseases in heterogeneous populations. J Math Biol 28:365–382
Diekmann O, Heesterbeek J A P, Roberts M G (2010) The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface 7:873–885
Eisenberg J N S, Desai M A, Levy K, Bates S J, Liang S, Naumoff K, Scott J C (2007) Environmental determinants of infectious disease: a framework for tracking causal links and guiding public health research. Environ Health Perspect 115:1216–1223
Eisenberg M C, Kujbida G, Tuite A R, Fisman D N, Tien J H (2013a) Examining rainfall and cholera dynamics in Haiti using statistical and dynamic modeling approaches. Epidemics 5:197–207
Eisenberg M C, Shuai Z, Tien J H, van den Driessche P (2013b) A cholera model in a patchy environment with water and human movement. Math Biosci 246:105–112
Emch M, Yunus M, Escamilla V, Feldacker C, Ali M (2010) Local population and regional environmental drivers of cholera in Bangladesh. Environ Health 9:2
Erlander S, Stewart N F (1990) The gravity model in transportation analysis—Theory and extensions. VSP Books, Zeist
Ferrière R, Gatto M (1995) Lyapunov exponents and the mathematics of invasion in oscillatory or chaotic populations. Theor Popul Biol 48:126–171
Gatto M, Mari L, Bertuzzo E, Casagrandi R, Righetto L, Rodriguez-Iturbe I, Rinaldo A (2012) Generalized reproduction numbers and the prediction of patterns in waterborne disease. Proc Natl Acad Sci USA 48:19703–19708
Gatto M, Mari L, Bertuzzo E, Casagrandi R, Righetto L, Rodriguez-Iturbe I, Rinaldo A (2013) Spatially explicit conditions for waterborne pathogen invasion. Am Nat 182:328–346
Gaudart J, Rebaudet S, Barrais R, Boncy J, Faucher B, Piarroux M, Magloire R, Thimothe G, Piarroux R (2013) Spatio-temporal dynamics of cholera during the first year of the epidemic in Haiti. PLoS Negl Trop Dis 7:e2145
Guerrant R L (1986) The global problem of amebiasis: current status, research needs, and opportunities for progress. Rev Infect Dis 8:218–227
Harris T E (1974) Contact interactions on a lattice. Ann Probab 2:969–988
Hashizume M, Armstrong B, Hajat S, Wagatsuma Y, Faruque A S G, Hayashi T, Sack D A (2008) The effect of rainfall on the incidence of cholera in Bangladesh. Epidemiology 19:103–110
Heesterbeek JAP, Roberts MG (1995a) Threshold quantities for helminth infections. J Math Biol 33:415–434
Heesterbeek J A P, Roberts M G (1995b) Threshold quantities for infectious diseases in periodic environments. J Biol Syst 4:779–787
Johnson A R, Hatfield C A, Milne B T (1995) Simulated diffusion dynamics in river networks. Ecol Model 83:311–325
Klausmeier C A (2008) Floquet theory: a useful tool for understanding nonequilibrium dynamics. Theor Ecol 1:153–161
Lipp E K, Huq A, Colwell R R (2002) Effects of global climate on infectious disease: the cholera model. Clin Microbiol Rev 15:757–770
Mari L, Bertuzzo E, Casagrandi R, Gatto M, Levin S A, Rodriguez-Iturbe I, Rinaldo A (2011) Hydrologic controls and anthropogenic drivers of the zebra mussel invasion of the Mississippi-Missouri river system. Water Resour Res 47:W03523
Mari L, Bertuzzo E, Righetto L, Casagrandi R, Gatto M, Rodriguez-Iturbe I, Rinaldo A (2012a) Modelling cholera epidemics: the role of waterways, human mobility and sanitation. J R Soc Interface 9:376–388
Mari L, Bertuzzo E, Righetto L, Casagrandi R, Gatto M, Rodriguez-Iturbe I, Rinaldo A (2012b) On the role of human mobility in the spread of cholera epidemics: towards an epidemiological movement ecology. Ecohydrology 5:531–540
Mari L, Casagrandi R, Bertuzzo E, Rinaldo A, Gatto M (2014) Metapopulation persistence and species spread in river networks. Ecol Lett 17:426–434
McMichael AJ, Woodruff RE, Hales S (2006) Climate change and human health: present and future risks. Lancet 367:859–869
Mendelsohn J, Dawson T (2008) Climate and cholera in KwaZulu-Natal, South Africa: the role of environmental factors and implications for epidemic preparedness. Int J Hyg Environ Health 211:156–162
Newman M E J (2005) Power laws, Pareto distributions and Zipf’s law. Contemp Phys 46:323–351
Pascual M, Rodó X, Ellner S P, Colwell R R, Bouma M J (2000) Cholera dynamics and El Niño Southern Oscillation. Science 289:1766–1769
Pascual M, Bouma M J, Dobson A P (2002) Cholera and climate: revisiting the quantitative evidence. Microbes Infect 4:237–245
Pascual M, Chaves L F, Cash B, Rodó X, Yunus M (2008) Predicting endemic cholera: the role of climate variability and disease dynamics. Clim Res 36:131–140
Reiner R C, King A A, Emch M, Yunus M, Faruque A S G, Pascual M (2012) Highly localized sensitivity to climate forcing drives endemic cholera in a megacity. Proc Natl Acad Sci USA 109:2033–2036
Righetto L, Casagrandi R, Bertuzzo E, Mari L, Gatto M, Rodriguez-Iturbe I, Rinaldo A (2012) The role of aquatic reservoir fluctuations in long-term cholera patterns. Epidemics 4:33–42
Righetto L, Bertuzzo E, Mari L, Schild E, Casagrandi R, Gatto M, Rodriguez-Iturbe I, Rinaldo A (2013) Rainfall mediations in the spreading of epidemic cholera. Adv Water Resour 60:34–46
Rinaldi S, Muratori S (1993) Conditioned chaos in seasonally perturbed predator-prey models. Ecol Model 69:79–97
Rinaldo A, Rodriguez-Iturbe I, Rigon R, Bras R, Ijjasz–Vasquez E, Marani A (1992) Minimum energy and fractal structures of drainage networks. Water Resour Res 28:2183–2195
Rinaldo A, Bertuzzo E, Mari L, Righetto L, Blokesch M, Gatto M, Casagrandi R, Murray M, Vesenbeckh S, Rodriguez-Iturbe I (2012) Reassessment of the 2010–2011 Haiti cholera outbreak and rainfall-driven multiseason projections. Proc Natl Acad Sci USA 109:6602–6607
Rinaldo A, Rigon R, Banavar J R, Maritan A, Rodriguez-Iturbe I (2014) Evolution and selection of river networks: statics,dynamics, and complexity. Proc Natl Acad Sci USA 111:2417–2424
Rodriguez-Iturbe I, Rinaldo A, Rigon R, Bras R, Ijjasz-Vasquez E, Marani A (1992) Fractal structures as least energy patterns-the case of river networks. Geophys Res Lett 19:889–892
Ruiz-Moreno D, Pascual M, Bouma M, Dobson A, Cash B (2007) Cholera seasonality in Madras. EcoHealth 4:52–62
Sardar T, Mukhopadhyay S, Bhowmick AR, Chattopadhyay J (2013) An optimal cost effectiveness study on Zimbabwe cholera seasonal data from 2008–2011. PLoS ONE 8:e81231
Tian J P, Wang J (2011) Global stability for cholera epidemic models. Math Biosci 232:31–41
Tien J H, Earn D J D (2010) Multiple transmission pathways and disease dynamics in a waterborne pathogen model. Bull Math Biol 72:1506–1533
Truscott J, Ferguson N M (2012) Evaluating the adequacy of gravity models as a description of human mobility for epidemic modelling. PLoS Comput Biol 8:e1002,699
Tuljapurkar S, Orzack S (1980) Population dynamics in variable environments. I. Long-run growth rates and extinction. Theor Popul Biol 18:314–342
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180:29–48
Wang W, Zhao X Q (2008) Threshold dynamics for compartmental epidemic models in periodic environments. J Dyn Diff Equat 20:699–717
World Health Organization (2008) The global burden of disease: 2004 Update. WHO Press, Geneva
World Health Organization (2010) Prevention and control of cholera outbreaks: WHO policy and recommendations. Tech. rep. World Health Organization, Regional Office for the EasternMediterranean
Zhang F, Zhao X Q (2007) A periodic epidemic model in a patchy environment. J Math Anal Appl 325:496–516
Zhou X, Cui J (2013) Threshold dynamics for a cholera epidemic model with periodic transmission rate. Appl Math Model 37:3093–3101
Zipf G K (1949) Human behavior and the principle of least effort: an introduction to human ecology. Addison-Wesley, Cambridge
Acknowledgments
The authors wish to thank two anonymous referees for their helpful comments on the manuscript. LM, EB and AR acknowledge the support provided by ERC advanced grant program through the project RINEC-227612 and by the SFN/FNS projects 200021 124930/1 and CR2312 138104/1. MG and AR acknowledge the support from the SFN/FNS project IZK0Z2 139537/1 for international cooperation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mari, L., Casagrandi, R., Bertuzzo, E. et al. Floquet theory for seasonal environmental forcing of spatially explicit waterborne epidemics. Theor Ecol 7, 351–365 (2014). https://doi.org/10.1007/s12080-014-0223-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-014-0223-y