Abstract
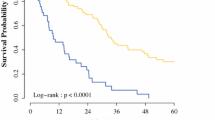
International guidelines exclude from surgery patients with peritoneal carcinosis of colorectal origin and a peritoneal cancer index (PCI) ≥ 16. This study aims to analyze the outcomes of patients with colorectal peritoneal carcinosis and PCI greater or equal to 16 treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) (CRS + HIPEC). We retrospectively performed a multicenter observational study involving three Italian institutions, namely the IRCCS Policlinico San Matteo in Pavia, the M. Bufalini Hospital in Cesena, and the ASST Papa Giovanni XXIII Hospital in Bergamo. The study included all patients undergoing CRS + HIPEC for peritoneal carcinosis from colorectal origin from November 2011 to June 2022. The study included 71 patients: 56 with PCI < 16 and 15 with PCI ≥ 16. Patients with higher PCI had longer operative times and a statistically significant higher rate of not complete cytoreduction, with a Completeness of Cytoreduction score (CC) 1 (microscopical disease) of 30.8% (p = 0.004). The 2-year OS was 81% for PCI < 16 and 37% for PCI ≥ 16 (p < 0.001). The 2-years DFS was 29% for PCI < 16 and 0% for PCI ≥ 16 (p < 0.001). The 2-year peritoneal DFS for patients with PCI < 16 was 48%, and for patients with PCI ≥ 16 was 57% (p = 0.783). CRS and HIPEC provide reasonable local disease control for patients with carcinosis of colorectal origin and PCI ≥ 16. Such results form the basis for new studies to reassess the exclusion of these patients, as set out in the current guidelines, from CRS and HIPEC. This therapy, combined with new therapeutical strategies, i.e., pressurized intraperitoneal aerosol chemotherapy (PIPAC), could offer reasonable local control of the disease, preventing local complications. As a result, it increases the patient’s chances of receiving chemotherapy to improve the systemic control of the disease.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Nassour I, Polanco PM (2017) Current management of peritoneal carcinomatosis from colorectal cancer: the role of cytoreductive surgery and hyperthermic peritoneal chemoperfusion. Curr Colorectal Cancer Rep 13:144–153
Kranenburg O, van der Speeten K, de Hingh I. (2021) Peritoneal Metastases From Colorectal Cancer: Defining and Addressing the Challenges (2021), Front Oncol. 11: 650098, doi:https://doi.org/10.3389/fonc.2021.650098
Sadeghi Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E et al (2000) Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88:358–363
Burnett et al. (2019) World Journal of Surgical Oncology, 17: 83
Sugarbaker P (1999) Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 43:S15–S25. https://doi.org/10.1007/s002800051093
Elias D, Gilly F, Boutitie F et al (2010) Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 28(1):63–68
Birgisson H, Enblad M, Artursson S, Ghanipour L, Cashin P, Graf W (2020) Patients with colorectal peritoneal metastases and high peritoneal cancer index may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (2020). Eur J Surg Oncol 46(12):2283–2291
Goéré D, Souadka A, Faron M, Cloutier AS, Viana B, Honoré C, Dumont F, Elias D (2015) Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study (1999). Ann Surg Oncol 22(9):2958–64. https://doi.org/10.1245/s10434-015-4387-5. (Epub 2015 Jan 29 PMID: 25631064)
Elias D, Blot F, El Otmany A et al (2001) Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer 92:71–76
de Boer NL, Brandt-Kerkhof ARM, Madsen EVE, Doukas M, Verhoef C, Burger JWA (2021) The accuracy of the surgical peritoneal cancer index in patients with peritoneal metastases of colorectal cancer (2021). Dig Surg 38(3):205–211. https://doi.org/10.1159/000513353. (Epub 2021 Mar 3 PMID: 33657551)
Quenet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D et al (2021) Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 22:256–266. https://doi.org/10.1016/S1470-2045(20)30599-4
AIOM (2021) Linee Guida Tumori Primitivi e Secondari del Peritoneo
Krebsgesellschaft D. S3-Leitlinie Kolorektales Karzinom, Langversion 2.1. (2019) Leitlinienprogramm Onkologie; Berlin
https://www.snfge.org/content/4-cancer-colorectal-metastatique
The National Institute for Health and Care Excellence (2020) NICE Guideline. The National Institute for Health and Care Excellence; London, Colorectal cancer
National Comprehensive Cancer Network (2021) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) NCCN; Plymouth Meeting, Colon Cancer, pp. MS-28–MS-30
Brind’Amour A, Dube P, Tremblay JF, Soucisse ML, Mack L, Bouchard-Fortier A, McCart JA, Govindarajan A, Bischof D, Haase E et al (2020) Canadian guidelines on the management of colorectal peritoneal metastases. Curr Oncol 27:e621–e631. https://doi.org/10.3747/co.27.6919
Steffen T, Eden J, Bijelic L, Glatzer M, Glehen O, Goere D, de Hingh I, Li Y, Moran B, Morris D et al (2020) Patient selection for hyperthermic intraperitoneal chemotherapy in patients with colorectal cancer: consensus on decision making among international experts. Clin Colorectal Cancer 19:277–284. https://doi.org/10.1016/j.clcc.2020.06.010
Cashin PH, Mahteme H, Spång N, Syk I et al (2016) Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: a randomized trial. Eur J Cancer 53:155–162
U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0 Published: May 28, 2009 (v4.03: June 14, 2010). Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06
Girshally R, Demtroder C, Albayrak N, Zieren J, Tempfer C, Reymond MA (2016) Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 14:253. https://doi.org/10.1186/s12957-016-1008-0
Alyami M, Mercier F, Siebert M, Bonnot PE, Laplace N, Villeneuve L, Passot G, Glehen O, Bakrin N, Kepenekian V (2021) Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 47:128–133. https://doi.org/10.1016/j.ejso.2019.06.028
Adamina M, Warlaumont M, Berger MD, Däster S, Delaloye R, Digklia A, Gloor B, Fritsch R, Koeberle D, Koessler T, Lehmann K, Müller P, Peterli R, Ris F, Steffen T, Weisshaupt CS, Hübner M (2022) Comprehensive treatment algorithms of the swiss peritoneal cancer group for peritoneal cancer of gastrointestinal origin. Cancers (Basel) 14(17):4275. https://doi.org/10.3390/cancers14174275.PMID:36077810;PMCID:PMC9454505
Balzano G, Guarneri G, Pecorelli N, Paiella S, Rancoita PMV, Bassi C et al (2020) Modelling centralization of pancreatic surgery in a nationwide analysis. Br J Surg 107(11):1510–9
Funding
No funding was received to assist with preparing this manuscript.
Author information
Authors and Affiliations
Contributions
PF: conceptualization, data collection, data analysis, writing; LA: conceptualization, review, editing; AM: writing, data collection; F.C., L.C., FDM, J.V., P.P., A.P.: review and editing; S.F., S.M., AR, G.S., MT, CV: data collection and review. All the authors reviewed the final version of the paper and gave final approval for submission.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The current work was considered exempt from institutional board review due to the use of de-personalized information and its retrospective nature.
Human participants
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Due to the retrospective nature, consent for participation in the study was waived, and no direct referral to patient data was evident.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fugazzola, P., Moroni, A., Agnoletti, V. et al. Should we exclude patients with peritoneal carcinosis of colorectal origin and high PCI from CRS + HIPEC?. Updates Surg 75, 1819–1825 (2023). https://doi.org/10.1007/s13304-023-01579-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-023-01579-4