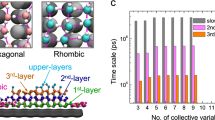
Abstract
The homogeneous crystallization of water at low temperature is believed to occur through the direct nucleation of cubic (Ic) and hexagonal (Ih) ices. Here, we provide evidence from molecular simulations that the nucleation of ice proceeds through the formation of a new metastable phase, which we name Ice 0. We find that Ice 0 is structurally similar to the supercooled liquid, and that on growth it gradually converts into a stacking of Ice Ic and Ih. We suggest that this mechanism provides a thermodynamic explanation for the location and pressure dependence of the homogeneous nucleation temperature, and that Ice 0 controls the homogeneous nucleation of low-pressure ices, acting as a precursor to crystallization in accordance with Ostwald’s step rule of phases. Our findings show that metastable crystalline phases of water may play roles that have been largely overlooked.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Eisenberg, D. & Kauzmann, W. The Structure and Properties of Water (Oxford Univ. Press, 1969).
Angell, C. A. Formation of glasses from liquids and biopolymers. Science 267, 1924–1935 (1995).
Mishima, O. & Stanley, H. The relationship between liquid, supercooled and glassy water. Nature 396, 329–335 (1998).
Debenedetti, P. Supercooled and glassy water. J. Phys. Condens. Matter 15, R1669–R1726 (2003).
Pruppacher, H. R. A new look at homogeneous ice nucleation in supercooled water drops. J. Atmos. Sci. 52, 1924–1933 (1995).
Rosenfeld, D. & Woodley, W. L. Deep convective clouds with sustained supercooled liquid water down to −37.5 °C. Nature 405, 440–442 (2000).
Koop, T., Luo, B., Tsias, A. & Peter, T. Water activity as the determinant for homogeneous ice nucleation in aqueous solutions. Nature 406, 611–614 (2000).
Soper, A. K. Structural transformations in amorphous ice and supercooled water and their relevance to the phase diagram of water. Mol. Phys. 106, 2053–2076 (2008).
Morishige, K. & Nobuoka, K. X-ray diffraction studies of freezing and melting of water confined in a mesoporous adsorbent (MCM-41). J. Chem. Phys. 107, 6965–6969 (1997).
Jelassi, J. et al. Studies of water and ice in hydrophilic and hydrophobic mesoporous silicas: pore characterisation and phase transformations. Phys. Chem. Chem. Phys. 12, 2838–2849 (2010).
Hansen, T., Koza, M. & Kuhs, W. Formation and annealing of cubic ice: I. Modelling of stacking faults. J. Phys. Condens. Matter 20, 285104 (2008).
Shilling, J. et al. Measurements of the vapor pressure of cubic ice and their implications for atmospheric ice clouds. Geophys. Res. Lett. 33, L17801 (2006).
Kobayashi, M. & Tanaka, H. Relationship between the phase diagram, the glass-forming ability, and the fragility of a water/salt mixture. J. Phys. Chem. B 115, 14077–14090 (2011).
Mayer, E. & Hallbrucker, A. Cubic ice from liquid water. Nature 325, 601–602 (1987).
Kohl, I., Mayer, E. & Hallbrucker, A. The glassy water–cubic ice system: A comparative study by X-ray diffraction and differential scanning calorimetry. Phys. Chem. Chem. Phys. 2, 1579–1586 (2000).
Murray, B. J. & Bertram, A. K. Formation and stability of cubic ice in water droplets. Phys. Chem. Chem. Phys. 8, 186–192 (2006).
Malkin, T. L., Murray, B. J., Brukhno, A. V., Anwar, J. & Salzmann, C. G. Structure of ice crystallized from supercooled water. Proc. Natl Acad. Sci. USA 109, 1041–1045 (2012).
Svishchev, I. M. & Kusalik, P. G. Crystallization of liquid water in a molecular dynamics simulation. Phys. Rev. Lett. 73, 975–978 (1994).
Matsumoto, M., Saito, S. & Ohmine, I. Molecular dynamics simulation of the ice nucleation and growth process leading to water freezing. Nature 416, 409–413 (2002).
Moore, E. B. & Molinero, V. Is it cubic? Ice crystallization from deeply supercooled water. Phys. Chem. Chem. Phys. 13, 20008–20016 (2011).
Li, T., Donadio, D., Russo, G. & Galli, G. Homogeneous ice nucleation from supercooled water. Phys. Chem. Chem. Phys. 13, 19807–19813 (2011).
Reinhardt, A., Doye, J. P., Noya, E. G. & Vega, C. Local order parameters for use in driving homogeneous ice nucleation with all-atom models of water. J. Chem. Phys. 137, 194504–194504 (2012).
Moore, E. & Molinero, V. Structural transformation in supercooled water controls the crystallization rate of ice. Nature 479, 506–508 (2011).
Zhao, Z. et al. Tetragonal allotrope of group 14 elements. J. Am. Chem. Soc. 134, 12362–12365 (2012).
Abascal, J. & Vega, C. A general purpose model for the condensed phases of water: TIP4P/2005. J. Chem. Phys. 123, 234505 (2005).
Sanz, E., Vega, C., Abascal, J. & MacDowell, L. Phase diagram of water from computer simulation. Phys. Rev. Lett. 92, 255701 (2004).
Jacobson, L. C., Hujo, W. & Molinero, V. Thermodynamic stability and growth of guest-free clathrate hydrates: a low-density crystal phase of water. J. Phys. Chem. B 113, 10298–10307 (2009).
Romano, F., Sanz, E. & Sciortino, F. Crystallization of tetrahedral patchy particles in silico. J. Chem. Phys. 134, 174502 (2011).
Ghiringhelli, L. M. et al. State-of-the-art models for the phase diagram of carbon and diamond nucleation. Mol. Phys. 106, 2011–2038 (2008).
Ten Wolde, P. R., Ruiz-Montero, M. J. & Frenkel, D. Numerical evidence for bcc ordering at the surface of a critical fcc nucleus. Phys. Rev. Lett. 75, 2714–2717 (1995).
Santra, M., Singh, R. S. & Bagchi, B. Nucleation of a stable solid from melt in the presence of multiple metastable intermediate phases: Wetting, Ostwald step rule and vanishing polymorphs. J. Phys. Chem. B 117, 13154–13163 (2013).
Lundrigan, S. E. & Saika-Voivod, I. Test of classical nucleation theory and mean first-passage time formalism on crystallization in the Lennard-Jones liquid. J. Chem. Phys. 131, 104503 (2009).
Sanz, E. et al. Homogeneous ice nucleation at moderate supercooling from molecular simulation. J. Am. Chem. Soc. 135, 15008–15017 (2013).
Stillinger, F. H. Water revisited. Science 209, 451–457 (1980).
Russo, J. & Tanaka, H. Understanding water’s anomalies with locally favored structures. Nature Commun. 5, 3556 (2014).
Ghiringhelli, L. M., Valeriani, C., Meijer, E. & Frenkel, D. Local structure of liquid carbon controls diamond nucleation. Phys. Rev. Lett. 99, 055702 (2007).
Kawasaki, T. & Tanaka, H. Formation of crystal nucleus from liquid. Proc. Natl Acad. Sci. USA 107, 14036–14041 (2010).
Russo, J. & Tanaka, H. The microscopic pathway to crystallization in supercooled liquids. Sci. Rep. 2, 505 (2012).
Russo, J. & Tanaka, H. Selection mechanism of polymorphs in the crystal nucleation of the Gaussian core model. Soft Matter 8, 4206–4215 (2012).
Tanaka, H. Bond orientational order in liquids: Towards a unified description of water-like anomalies, liquid–liquid transition, glass transition, and crystallization. Eur. Phys. J. E 35, 1–84 (2012).
Seidl, M., Amann-Winkel, K., Handle, P. H., Zifferer, G. & Loerting, T. From parallel to single crystallization kinetics in high-density amorphous ice. Phys. Rev. B 88, 174105 (2013).
Marchand, D. J., Hsiao, E. & Kim, S. H. Non-contact AFM imaging in water using electrically-driven cantilever vibration. Langmuir 29, 6762–6769 (2013).
Lied, A., Dosch, H. & Bilgram, J. H. Surface melting of ice Ih single crystals revealed by glancing angle X-ray scattering. Phys. Rev. Lett. 72, 3554–3557 (1994).
Vega, C., Sanz, E., Abascal, J. & Noya, E. Determination of phase diagrams via computer simulation: Methodology and applications to water, electrolytes and proteins. J. Phys. Condens. Matter 20, 153101 (2008).
Steinhardt, P. J., Nelson, D. R. & Ronchetti, M. Bond-orientational order in liquids and glasses. Phys. Rev. B 28, 784–805 (1983).
Lechner, W. & Dellago, C. Accurate determination of crystal structures based on averaged local bond order parameters. J. Chem. Phys. 129, 114707 (2008).
Auer, S. & Frenkel, D. Prediction of absolute crystal-nucleation rate in hard-sphere colloids. Nature 409, 1020–1023 (2001).
Acknowledgements
We are grateful to F. Sciortino for a critical reading of the manuscript and to J. Doye and A. Reinhardt for useful discussions. This study was partly supported by Grants-in-Aid for Scientific Research (S) and Specially Promoted Research from the Japan Society for the Promotion of Science (JSPS), the Aihara Project, the FIRST programme from JSPS, initiated by the Council for Science and Technology Policy (CSTP), a JSPS short-term fellowship for F.R., and a JSPS Postdoctoral Fellowship for J.R.
Author information
Authors and Affiliations
Contributions
J.R. and F.R. performed the numerical simulations and the data analysis. H.T. proposed and supervised the study. All authors discussed the results and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2172 kb)
Rights and permissions
About this article
Cite this article
Russo, J., Romano, F. & Tanaka, H. New metastable form of ice and its role in the homogeneous crystallization of water. Nature Mater 13, 733–739 (2014). https://doi.org/10.1038/nmat3977
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3977
This article is cited by
-
Unsupervised topological learning approach of crystal nucleation
Scientific Reports (2022)
-
Probing the critical nucleus size for ice formation with graphene oxide nanosheets
Nature (2019)
-
Room temperature electrofreezing of water yields a missing dense ice phase in the phase diagram
Nature Communications (2019)
-
Mapping uncharted territory in ice from zeolite networks to ice structures
Nature Communications (2018)
-
Experimental and Theoretical Justification of Designing a Cable Line Exposed to Cryogenic Cracking
Soil Mechanics and Foundation Engineering (2018)