Abstract
Here, we present the results related to a new unique terrestrial ecosystem found in an englacial hypersaline brine found in Northern Victoria Land (Antarctica). Both the geochemistry and microbial (prokaryotic and fungal) diversity revealed an unicity with respect to all the other known Antarctic brines and suggested a probable ancient origin mainly due a progressive cryoconcentration of seawater. The prokaryotic community presented some peculiarities, such as the occurrence of sequences of Patescibacteria (which can thrive in nutrient-limited water environments) or few Spirochaeta, and the presence of archaeal sequences of Methanomicrobia closely related to Methanoculleus, a methanogen commonly detected in marine and estuarine environments. The high percentage (35%) of unassigned fungal taxa suggested the presence of a high degree of undiscovered diversity within a structured fungal community (including both yeast and filamentous life forms) and reinforce the hypothesis of a high degree of biological uniqueness of the habitat under study.
Similar content being viewed by others
Introduction
Interest in brines within cryoenvironments has increased after they have been found on Mars (e.g.1,2). Brines were also discovered in the deep subsurface in Canada, Finland, Germany and Sweden. In Antarctica, hypersaline brines were found at McMurdo Sound, within the permafrost of the Taylor Valley (e.g.3), as well as below ice-sealed Antarctic lakes (e.g.4) or in the Antarctic subglacial lakes (i.e.5). Several studies modeled the occurrence and the effects of the subglacial brines or detected their occurrence through indirect methods (e.g.3,6,7,8). Despite these efforts the subglacial aquatic systems remain poorly understood mainly due to the lack of direct sampling. Englacial brines that flow within glaciers9, ice sheets or ice shelves10 are even less known. The connections between englacial and subglacial brines and their origins are still debated although at least in Taylor Valley several studies (i.e.3) provided strong inputs to their comprehension. Different mechanisms regarding the origin of the hypersaline brines were proposed (e.g.9,11). Subglacial and endoglacial brines can be extremely important in regard to glacier dynamics (e.g.12,13) and even more for ecological interest (e.g.14). Microbial communities sharing subglacial environment have been studied in recent decades, especially in order to understand possible effects on the weathering of the underlying rock (e.g.15,16,17). Probably, the most studied case is the outflow brine of Blood Falls in Taylor Valleys (e.g.18,19,20) that revealed the presence of a thriving community of chemosynthetic bacteria whose 74% of clones and isolates shared high 16S rRNA gene sequence homology with phylotypes from marine systems.
The microbial communities of hypersaline brines observed below ice-sealed Antarctic lakes were surely more investigated (i.e.21,22,23,24,25,26,27,28,29); accordingly, they have been used as comparison in this paper. The phylogenetic groups found in the different frozen lakes were quite different each other and exhibited different dominant groups: e.g. Bacteroidetes and Actinobacteria in East Bonney Lake or Proteobacteria (and Cyanobacteria26) in Lake Vanda27.
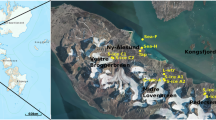
Here we described the biotic and abiotic unicity of a hypersaline flowing endoglacial brine sampled through a borehole cored during the austral summer 2019 on the Boulder Clay Glacier, a coastal cold based glacier not far from the Italian Antarctic station (Mario Zucchelli Station, MZS, 74° S, Fig. 1). This study enhances the importance and the unicity of an endoglacial ecosystem that differs from all the others known to date, and opens a provocative question on the origin of the brine and its related ecosystem.
(A) Location map of the study area. (B) georeferenced satellite image with the location of the borehole (BC), the Italian Antarctic Station Mario Zucchelli (MZS) and some localities cited in the text. (C) Stratigraphy of the core (a, snow and firn; b) milky bubbly ice; c) bubbly ice with few clasts; d) well sorted frozen sand layer; e) clean ice with few clasts; f) ice with vertical tubules; g) ice with vertical tubules and black inclusions; h) clean ice; i) salty ice; l) salty slush (8.62–8.72) and brine (9.1–9.3); m) frozen till. (D) View of the borehole location from W to E.
Results
The endoglacial hypersaline brine system
The brines were found at 9.1 m of depth within a valley glacier characterized by a glacial unconformity and an erosional channel at 3 m of depth in which thin alluvial sediments (3 cm thick) were found. These peculiar structures are described in Forte et al.30. The ice below the unconformity appears almost similar down to 7.9 m of depth where it became slightly salty until 8.62 m where started a layer of salty, yellowish slush that at 9.1 m of depth became completely liquid (brine) at the date of sampling (26 November 2019) and remained liquid and flowing also at the last check (7 December 2019) when the level of brines raised upward 2.25 m. In situ the brine appeared quite rich in gas bubbles, with a temperature of − 17.4 °C, slightly basic (from 7.4 and 7.6), hypersaline (NaCl concentration is about 198 g L−1) and with a low percentage of DO (12.9%). The geochemical characteristics of the two samples of the same brine (BC-1 and BC-2) are reported in Table S1. The chemical analysis showed high contents in chlorides (3.8 eq L−1), sodium (2.7 eq L−1), magnesium (1.1 eq L−1) and potassium (110 meq L−1) with values that are close to only a few other Antarctic brines, such as the East Lake Bonney (ELB)31 and lake Vida brines (VD)21 although BC brines slightly exceed in sodium and potassium with respect to ELB and Lake Vanda (LV). Differently, sulfates (60 meq L−1) and calcium (160–170 meq L−1) showed values that are in line with the majority of the other Antarctic brines (e.g.9,21,22,31). The chemical peculiarity of BC brines with respect to the other Antarctic ones was preliminarily evaluated though Principal Component Analysis (PCA), including the six major ion concentrations as variables (see Tables S2–S4 for further details). Figure 2A shows the biplot of the first two PCs that explain together the 85% of the total variance. The PC1 (59%) is characterized by negative loadings of Mg2+, Na+, K+ and Cl− (see Table S3), while PC2 (26%) has positive/negative loadings of SO4/Ca (0.886/− 0.783, respectively). PC1 significantly correlates with the ionic strength of the brines (see Table S1) with a r-Pearson correlation coefficient of − 0.91 (p value < 10–6). Therefore, PC1 can be interpreted as indicative of the ionic activity of the brine solution. ELB, BC and, to a lesser extent, VD brines have the more negative PC1 values. The PC2, instead, indicates the main characteristics of the less saline LV and Tarn Flat (TF) brines, characterized by a more significant input of Ca2+ and sulfates, respectively. Figure 2B shows the Mg/K ratio vs Cl/SO4 of several Antarctic brines, where it is possible to observe that Cl/SO4 of BC brines is approximately 10 times higher than seawater (SW), but similar to ELB brines. Conversely, Mg/K ratio in BC is almost the same as the seawater and it is sensibly lower than the Englacial and Blood Falls brines that were the most similar to BC for this parameter.
Another peculiarity of BC brines (see Table S1) is the total N content of about 42–43 mM, consistently higher than other brines. It must be noted that we did not detect any significant signal due to NO2 or NH4+, while the overall contribution of inorganic nitrogen was due to NO3. Similarly, to what was found by Lyons et al.9, we suppose that this value is an oxidation artifact due to the storage before the analysis. Moreover, the determination of the total N with the independent analysis carried out with Elemental Analyzer, provided values similar to NO3. Therefore, we prefer to report the total N concentration without any chemical speciation.
The microbial diversity
Prokaryotic diversity
Analysis of amplicon sequencing variants (ASVs) revealed a total number of 1042 ASVs, which were then resolved in 25 prokaryotic phyla, with a resolution of 162 genera. Unclassified sequences were on average 6.9 and 12.9% at phylum and genus levels, respectively.
Archaea were present in a low percentage (0.5%) almost entirely affiliated to the genus Methanoculleus (87%). Eight bacterial phyla occurred at a percentage greater than 0.1%, namely Actinobacteria, Bacteroidetes, Cyanobacteria, Firmicutes, Patescibacteria, Planctomycetes, Proteobacteria and Verrucomicrobia. Among them, Proteobacteria, Bacteroidetes, Verrucomicrobia, Patescibacteria, and Actinobacteria exceeded 1%. In particular, Proteobacteria (average percentage 48.6%) were particularly abundant and mainly represented by Gammaproteobacteria (family Marinobacteraceae) and, to a lesser extent, Alphaproteobacteria. The second most abundant group was that of Bacteroidetes (27.6%; mainly Flavobacteriaceae), followed by Verrucomicrobia, Patescibacteria and Actinobacteria (7.1, 4.5 and 3.2%, respectively). Firmicutes, Planctomycetes and Cyanobacteria were in the range 0.3–0.7%.
At genus level, Marinobacter (among Proteobacteria) was predominant (38.5%), followed at a lesser extent by Psychroflexus and Flavimarina (8.3 and 5.1%, respectively; among Bacteroidetes), Psychrobacter (7.8%; among Proteobacteria), and Luteolibacter (5.5%; among Verrucomicrobia). Other genera occurring at a percentage between 1 and 5% were Gillisia, Salegentibacter, Aurantivirga, Mesonia and Algoriphagus, all among Bacteroidetes (63.8% of Bacteroidetes sequences were resolved at genus level). Ten genera were found among Actinobacteria, but none of them exceeded 1%. No genus was determined among Patescibacteria (Fig. 3).
Fungal diversity
A total of 141 ASVs belonging to three different fungal phyla were found. A high percentage (> 25%) of ASVs was assigned to unclassified fungi. At phylum level, 56.71% of ASVs were represented by Ascomycota, followed by Basidiomycota with 8.2%, and Mucoromycota with 1%. At genus level, the most represented taxa were Cladosporium (10%), Phoma (9%), Penicillium (8%), Phaeoisaria (4%), Aspergillus (3%), and to a lesser extent Cyphellophora (1.5%), Periconia (1.2%), Parengyodontium (1%), and Mucor (1%). Considering yeast life forms, Glaciozyma was the unique yeast genus exhibiting an abundance > 1%. In addition, several rare genera (< 1%) were grouped as others (14%). The analysis of the fungal growth morphology showed that the brine was characterized mainly by filamentous fungi life forms (55.56%), followed by yeasts (8.44%) and black yeast − like fungi (1.23%) (Fig. 4).
Discussion
Endoglacial brines as a unique ecosystem for microbial life
To date, researchers have mainly focused their attention on prokaryotic diversity of Antarctic brines18,23,24,25, while fungal diversity has not yet been explored extensively22,26. Regarding the endoglacial brines of Vida, Vanda and Bonney (west and east lobes) lakes, a number of papers have been published since the last 1990s on the biotic and abiotic characteristics e.g.21,27,28,29. Even in this case, however, the study of microbial communities was almost exclusively aimed at the analysis of the prokaryotic fraction.
The occurrence of halophiles has been documented in worldwide hypersaline lakes: most of them belong to the phyla Proteobacteria, Cyanobacteria, Rhodothermaeota, Firmicutes, Actinobacteria, Bacteroidetes and Spirochaetes32. However, phylogenetically the prokaryotic assemblage of BC brine showed both similarities and divergences with other Antarctic hypersaline systems. A number of phylogenetic groups (such as Cyanobacteria and Planctomycetes) that are widespread in freshwater and marine ecosystems were observed in BC brines, as well as in East Bonney and Vanda Lakes, but they were not detected in Lake Vida. Differently from East Bonney Lake, where Bacteroidetes and Actinobacteria were predominant26, Proteobacteria dominated within the bacterial communities of BC brines. The dominance of Proteobacteria (and Cyanobacteria) was also reported by Ramoneda et al.33 in Lake Vanda. However, this result referred to microbial mats, whereas water under the ice cover of the lake was characterized by a high relative abundance of Actinobacteria. In the East Bonnie Lake, Proteobacteria were the next most abundant phylum after Bacteroidetes and Actinobacteria, and mainly consisted of Betaproteobacteria, which were instead absent in BC brines.
Gammaproteobacteria also occurred in the hypersaline East Bonney Lake, becoming dominant only in its deeper layers29, and in Lake Vanda34, thus highlighting their adaptation to high salinity levels. Consistently with Lake Vanda, the proteobacterial fraction of BC brines prokaryotic community included Alpha- and Gammaproteobacteria, whereas Deltaproteobacteria were not detected. The prokaryotic assemblage of BC brines also distinguished from those of Lake Vida and Blood Falls, as well as from East Bonney and Vanda Lakes, because it contains Patescibacteria as an exclusive phylotype. Patescibacteria (as well as Verrucomicrobia and Actinobacteria) are generally reported as bacterial inhabitants of permafrost (e.g.35,36,37). Interestingly, members of Patescibacteria can thrive in nutrient-limited water environments having simplified genomes which drive functions essential to growth and reproduction and retain stress response systems38. Further, the prokaryotic assemblage of BC brines (as observed for Lake Vida) harbored few Spirochaeta-related sequences unlike those of the Blood Falls brine and East Bonney Lake. These microorganisms and their metabolic features (such as H2 utilization, EPS production and fermentation) may be useful to describe the functional ecology of briny systems.
Another noteworthy feature of the BC brines concerned the occurrence, albeit scarce, of Archaea (not detected, for example, in Lake Vida). Significant diverse archaeal populations were reported for cold briny habitats of marine origin, such as the Vestfold Hills lake system in Eastern Antarctica39, and Lake Vanda34. Differently from observations on Lake Vanda, sequences of Methanomicrobia detected in BC brines were most closely related to Methanoculleus (instead of the methylotrophic Methanomassiliicoccus34), a genus of methanogens commonly detected in marine and estuarine environments (e.g. shallow sediments40), but rarely reported for lacustrine habitats (e.g.41). These hydrogenotrophic methanogens well adapt to low H2 concentration, therefore possibly having an advantage over other methanogens in saline environments. Based on this result, Methanoculleus representatives are the most likely active methanogens in BC brines. Thus, differently from Lake Vanda brine, methanogenesis in BC brines occurs by the most widespread pathway, i.e. hydrogenotrophic methanogenesis, which has been suggested to be the ancestral form of methane production42.
At genus level, the gammaproteobacterial Marinobacter and Psychrobacter lineages are cosmopolitan and ecologically relevant in icy brines (e.g.20,21). The predominance of sequences related to the Marinobacter genus makes the prokaryotic assemblage of BC brines highly similar to those reported for brines of the Lake Vida and Blood Falls21. Marinobacter members were also abundant at 30 m-depth of the hypersaline East Bonney Lake29. Marinobacter spp. are aerobic with a strictly respiratory type of metabolism. They can grow anaerobically by denitrification coupled to the oxidation of a suitable donor carbon substrate and Na+ is required for growth. Among Bacteroidetes, the genus Psychroflexus is found within moderately hypersaline ecosystems across the world, including sea-ice43, salt lake (e.g.44,45), salt pan46 and marine solar saltern47. Members of this genus are generally aerobic, slightly or moderately halophilic.
Considering fungal communities, despite the presence of halophilic fungi in hypersaline systems were documented32, the almost total absence of analyses aimed at studying the eukaryotic fraction makes difficult (or even impossible) any comparison with the other endoglacial brines found in the Vida, Vanda and East Bonney lakes. The sole comparison that can be made is that with the study of Murray et al.21, who reported that PCR surveys to detect both eukaryal and archaeal SSU rRNA genes from genomic DNA were negative, thus assuming the absence of eukaryotic microbial populations (including both yeast and fungal life forms) in endoglacial brines of Vida lake. This result underlines the strong difference between them and the BC brines, where the presence of a structured fungal community was found.
In detail, the percentage of filamentous fungi found was higher (55.56%) than other growth morphology (i.e. yeasts and black yeast-like fungi), differently from the brine sampled in a frozen lake of Tarn Flat where yeasts dominated the fungal diversity22, and in another frozen lake of Boulder Clay, where the percentage of filamentous fungi and yeast form was similar26. Moreover, the high percentage of unassigned taxa (35%) found in the BC brines suggests the presence of high undiscovered fungal diversity, reinforcing the hypothesis of a high degree of biological uniqueness.
Although some of them can be found in extreme habitats worldwide48,49, the most abundant filamentous fungal genera colonizing the BC brines (Aspergillus, Cladosporium and Penicillium), as well as some minor genera (Engyodontium, Phoma), have been currently found in Antarctic seawaters and marine-associated habitats (e.g.50,51). Likewise, Glaciozyma, which is the unique yeast genus exhibiting an abundance ≥ 1%, has been widely recovered in seawaters and marine-associated habitats50,51. It was also found in Antarctic brines from Tarn Flat22. On the basis of these results, and in analogy to what is reported above for bacterial taxa, the hypothesis of possible marine origin of the BC brines cannot be excluded.
Black yeast-like fungi exhibit morphological and physiological characteristics making them the organisms well adapted to the harsh Antarctic conditions. This microbial group is characterized by wide environmental plasticity and their ability to shift from one growth form to another, according to the physicochemical environmental conditions, may be regarded as an adaptive strategy to stressful conditions (e.g.52,53). Their lower abundance in BC brines compared to filamentous fungi and yeasts is compatible with their lower growth rates, as reported by Canini et al.54. However, the presence of some melanized fungi (i.e. members of the genera Aureobasidium, Hortaea, Phaeotheca and Trimmatostroma) have been found in hypersaline environments worldwide32.
BC endoglacial brines: a provocative question about their origin?
It is now widely accepted that hypersaline surface or subsurface waters (brines) have been pervasive on Mars, at least periodically, throughout the last 3.5 billion years, and may be present still today1,55,56,57,58.
Conversely hypersaline waters are not so common on Earth. However, in Antarctica that is considered the best Mars analogue for many reasons, these hypersaline waters are likely more widespread, at least in the Dry Valleys.
The hypersalinity of Antarctic brines has been explained through different mechanisms, although there is consensus that coastal Antarctic brines mainly come from the cryoconcentration of seawater59,60. Therefore, their chemistry is only scarcely influenced by rock-weathering and/or biogeochemical cycles contributions61 and the possible input deriving from the dissolution of halites is poorly convincing in East Antarctica62. The cryoconcentration of seawater provides the preferential precipitation of the less soluble sodium sulfate decahydrates (mirabilite) at temperature lower than − 8.2 °C. This mechanism is considered responsible for the depletion of Na+ and SO42−. However, as observed by Cragin et al.59, the Na/SO4 ratio in brines is lower than in seawater, mainly due to the major relative changes in SO42− with respect to Na+, while Na2SO4 precipitates. In accordance with Cragin et al.59 observations, BC brines show a Na/SO4 ratio (meq/meq) of 0.022 that is about 5-times lower than seawater (i.e. 0.12). The effect of the cryoconcentration is also evident from the Cl/SO4 values reported in Fig. 2B, where the higher Cl/SO4 found in BC and ELB are clearly indicative of an enhanced cryoconcentration process. In addition, Mg/K ratios, here proposed as indicators of input from non-marine sources, such as weathering, rock-water interactions as well as biogeochemical cycles, shows BC values very close to seawater composition, while ELB provides values significantly higher. Therefore, the hypersalinity of BC brines is mainly originated by seawater after an intensive cryoconcentration that likely began to occur already in remote epochs. The other peculiarity of the BC brines is the high N content that together with the total P, resulted significantly higher than other brines. This occurrence suggests a prolonged or more intense biological activity in BC brines with respect to the other ones considered in this study. Considering the advanced seawater cryoconcentration and the very scarce contribution of rock weathering in conjunction with the probable more prolonged and/or more intense biological activity, the question about where and when these brines were generated arises. At this stage, we cannot provide a comprehensive answer, but we can attempt to formulate the following innovative hypothesis. Indeed, Lyons et al.9 have already explained that the end-member brine found below the Taylor glacier (Blood Falls), was derived from seawater and they speculated on the mechanism controlling cryoconcentration that led to the formation of the hypersaline water.
Differently, in our case the actual landscape does not show any evidence of ancient lakes or fjords or inland sea where seawater could be present in the remote past. In addition, we have to consider that according to Armienti and Baroni63, the glaciers of this sector of Victoria Land have preserved typical polar geomorphological features with negligible erosional power, during the last 8.2–7.5 millions of years. Apparently only the higher peaks of the Northern Foothills as the close Mt. Abbott (Fig. 1) were deglaciated since the mid Pliocene (3.85 Ma; Di Nicola et al.64) while, since that time, areas below 720 m asl have been repeatedly exposed and overridden by expanding ice bodies. On the other hand, if we consider Levy et al.65 at the end of the mid Pliocene, after the peak of the Pliocene warmth, when global average temperatures increased to 4 °C warmer than pre-industrial levels, a phase of tectonic uplift occurred. Consequently, the areas containing seawater and marine sediments (lagoon conditions?), where brines could have been formed, rose up. Although it is not possible to exclude that the origin of the brine can predate the Miocene, where, in some depressions, brines might had been preserved by the glacial erosion before the 8.2 Ma, even during the Early and Late Oligocene, when the Victoria Land coast was characterized by the presence of vegetation, such as Nothofagus spp., podocarps and bryophytes66.
Methods
Boulder Clay Glacier is located (BC, Fig. 1) just a few kilometers from the Mario Zucchelli Italian Station (MZS). The glacier reaches the sea at Adelie Cove and eastward is limited by a debris-covered glacier that flows towards SSE. The borehole BC (red star in Fig. 1b) reached the brines at 9.1 m of depth. The borehole stratigraphy is represented in Fig. 1c. The measurements in situ were carried out with a multiparametric probe (Hanna Instruments—HI98194 model) a few minutes after the coring. Two BC brines (BC-1 and BC-2) were sampled with a peristaltic pump using sterile tubes in the same day at only a couple of minutes of distance. The samples were immediately transported to the labs of MZS and preserved at − 20 °C and successively in Italy at Messina Labs keeping the − 20 °C temperature during all the transport.
The chemical analyses of BC brines were carried out in the laboratory of Venice. All liquid brine samples were filtered using a PTFE membrane (pore size 0.45 µm) before analyses. The anions (NO3−, SO42− and Cl−) and cations (NH4+, Na+, K+, Mg2+ and Ca2+) were analyzed using ion chromatography (Metrohm 761 Compact IC Chromatography) equipped with a Cation 1–2 (particle size 7 μm; eluent: HNO3 3 mM) and a Metrosep Anion supp/4 (particle size 5 μm; eluent: HCO3−/CO32− buffer 1.7/1.8 mM) column for cation and anion analysis, respectively. The brines were appropriately diluted in ultra-pure water to fit the calibration range and analyzed by ICP-MS using an iCAP RQ (Thermo Scientific) instrument equipped with an ASX-560 autosampler (Teledyne Cetac Technologies), PFA cyclonic spray chamber at 2.7 °C, sapphire injector, quartz torch, Ni cones and 1550 W of plasma radio frequency power. The ICP analysis was performed for Na, K, Ca, Mg and P. The Na, K, Mg and Ca values obtained with ICP-MS were not significantly different from those obtained with cationic IC and therefore we reported a mean value of the results obtained with these two techniques for these elements.
Total carbon and nitrogen were determined with a Flash 2000 HT Elemental Analyzer (Thermo Scientific). The principal component analysis was carried out with R Studio, where the variables (Na, K, Mg, Ca, SO4 and Cl) were preliminarily scaled.
DNA extraction and NGS sequencing
For microbiological analyses, collected BC brines were pooled in a single sample and filtered (between 300 and 350 ml) in five replicates on polycarbonate filters (size 45 mm; porosity 0.22 µm). DNA was extracted from membranes using the Power Soil DNA extraction kit (MoBio Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions. DNA concentrations and purity were quantified by using a NanoDrop ND-1000 UV–Vis spectrophotometer (NanoDrop Technologies, USA). Bacterial 16S rDNA region (V3–V4) and fungal internal transcribed spacer region 2 (ITS2) were amplified using the following primers:
-
i)
IlluAdp_16S_341f 5′-CCTACGGGNGGCWGCAG-3′ and IlluAdp_16S_805r 5′-GGACTACHVGGGTATCTAATCC-3′ for bacterial 16S rRNA gene region V3–V4.
-
ii)
IlluAdp_ITS31_NeXTf 5′-CATCGATGAAGAACGCAG-3′ and IlluAdp_ITS4_NeXTr5′-TCCTCCGCTTATTGATATGC-3′67 for fungi.
Sequencing was performed using the Illumina MiSeq platforms, following the standard protocols of the company IGA Technology Services Srl (Udine, Italy).
Bioinformatics analysis
FastQC was used to check the quality of raw sequences68. Sequences were pre-processed, quality filtered, trimmed, de-noised, merged, modeled, and analyzed via DADA2 within QIIME269 and chimeras were removed following the ‘consensus’ method reported by Callahan et al.70.
Bacterial taxonomy annotation was performed using Silva 138 99% ASVs full-length sequences (silva-138-99-nb-classifier.qza). Fungal taxonomy annotation was performed using a Naïve–Bayes classifier trained on the UNITE + INSD database against the representative sequences71. All sequences have been submitted to the National Center for Biotechnology Information (NCBI) under the BioProject PRJNA826749, with the biosample accession numbers SAMN27582456, SAMN27582457, SAMN27582458, SAMN27582459 and SAMN27582460 for bacteria, and SAMN27584063, SAMN27584064, SAMN27584065, SAMN27584066 and SAMN27584067 for fungi.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Martínez, G. M. & Renno, N. O. Water and brines on Mars: Current evidence and implications for MSL. Sp. Sci. Rev. 175(1), 29–51 (2013).
Orosei, et al. Radar evidence of subglacial liquid water on Mars. Science 361(6401), 490–493. https://doi.org/10.1126/science.aar7268 (2018).
Mikucki, J. A. et al. Deep groundwater and potential subsurface habitats beneath an Antarctic dry valley. Nat. Commun. 6(6831), 1–9 (2015).
Forte, E., Dalle Fratte, M., Azzaro, M. & Guglielmin, M. Pressurized brines in continental Antarctica as a possible analogue of Mars. Sci. Rep. 6, 33158 (2016).
Siegert, M. J., Kennicutt, M. C. & Bindschadler, R. A. Antarctic Subglacial Aquatic Environments (Wiley, 2013).
Boulton, G. S., Caban, P. E. & van Gijssel, K. Groundwater flow beneath ice sheets: Part I—Large-scale patterns. Quatern. Sci. Rev. 14, 545–562 (1995).
Fricker, H. A., Carter, S. P., Bell, R. E. & Scambos, T. Active lakes of Recovery Ice Stream, East Antarctica: A bedrock-controlled subglacial hydrological system. J. Glaciol. 60(223), 1015–1030. https://doi.org/10.3189/2014JoG14J063 (2014).
Siegert, M. J. A wide variety of unique environments beneath the Antarctic ice sheet. Geology 44(5), 399–400. https://doi.org/10.1130/focus052016.1 (2016).
Lyons, W. B. et al. The geochemistry of englacial brine from Taylor Glacier, Antarctica. J. Geophys. Res. Biogeosci. 124, 633–648. https://doi.org/10.1029/2018JG004411 (2019).
Campbell, S., Courville, Z., Sinclair, S. & Wilner, J. Brine, englacial structure and basal properties near the terminus of McMurdo Ice Shelf, Antarctica. Ann. Glaciol. 58, 74. https://doi.org/10.1017/aog.2017.26 (2017).
Greene, S. et al. Canadian Shield brine from the Con Mine, Yellowknife, NT, Canada: Noble gas evidence for an evaporated Palaeozoic seawater origin mixed with glacial meltwater and Holocene recharge. Geochim. Cosmochim. Acta 72, 4008–4019. https://doi.org/10.1016/j.gca.2008.05.058 (2008).
Siegfried, M. R., Fricker, H. A., Carter, S. P. & Tulaczyk, S. Episodic ice velocity fluctuations triggered by a subglacial flood in West Antarctica. Geophys. Res. Lett. 43, 2640–2648. https://doi.org/10.1002/2016GL067758 (2016).
Stearns, L. A., Smith, B. E. & Hamilton, G. S. Increased flow speed on a large East Antarctic outlet glacier caused by subglacial floods. Nat. Geosci. 1(12), 827–831. https://doi.org/10.1038/ngeo356 (2008).
Kennicutt, M. C. et al. A roadmap for Antarctic and Southern Ocean science for the next two decades and beyond. Antarct. Sci. 27(01), 3–18. https://doi.org/10.1017/S0954102014000674 (2015).
Welch, K. A. et al. Spatial variations in the geochemistry of glacial meltwater streams in the Taylor Valley, Antarctica. Antarct. Sci. 22(06), 662–672. https://doi.org/10.1017/S0954102010000702 (2010).
Skidmore, M., Tranter, M., Tulaczyk, S. & Lanoil, B. Hydrochemistry of ice stream beds—evaporitic or microbial effects?. Hydrol. Process. 24(4), 517–523 (2010).
Lüttge, A. & Conrad, P. G. Direct observation of microbial inhibition of calcite dissolution. Appl. Environ. Microbiol. 20, 1627–1632 (2004).
Mikucki, J. A. & Priscu, J. C. Bacterial diversity associated with Blood Falls, a subglacial outflow from the Taylor Glacier, Antarctica. Appl. Environ. Microbiol. 73(12), 4029–4039 (2007).
Mikucki, J. A. et al. A contemporary microbially maintained subglacial ferrous “Ocean”. Science 324(5925), 397–400. https://doi.org/10.1126/science.1167350 (2009).
Chua, M. J. et al. Genomic and physiological characterization and description of Marinobacter gelidimuriae sp. Nov., a psychrophilic, moderate halophile from Blood Falls, an Antarctic subglacial brine. FEMS Microbiol. Ecol. 94, fiy021 (2018).
Murray, A. E. et al. Microbial life at −13 °C in the brine of an ice-sealed Antarctic lake. PNAS 109, 20626–20631. https://doi.org/10.1073/pnas.1208607109 (2012).
Borruso, L. et al. A thin ice layer segregates two distinct fungal communities in Antarctic brines from Tarn Flat (Northern Victoria Land). Sci. Rep. 8, 1–9 (2018).
Papale, M. et al. Microbial assemblages in pressurized Antarctic brine pockets (Tarn Flat, Northern Victoria Land): A hotspot of biodiversity and activity. Microorganisms 7, 333 (2019).
Azzaro, M. et al. The prokaryotic community in an extreme Antarctic environment: The brines of Boulder Clay lakes (Northern Victoria Land). Hydrobiologia 848, 1837–1857. https://doi.org/10.1007/s10750-021-04557-2 (2021).
Lo Giudice, A. et al. Prokaryotic diversity and metabolically active communities in brines from two perennially ice-covered Antarctic lakes. Astrobiology 21, 551–565 (2021).
Sannino, C. et al. Intra-and inter-cores fungal diversity suggests interconnection of different habitats in an Antarctic frozen lake (Boulder Clay, Northern Victoria Land). Environ. Microbiol. 22, 3463–3477 (2020).
Bratina, B. J., Stevenson, B. S., Green, W. J. & Schmidt, T. M. Manganese reduction by microbes from oxic regions of the lake vanda (Antarctica) water column. Appl. Environ. Microbiol. 64, 3791–3797 (1998).
Tregoning, G. S. et al. A halophilic bacterium inhabiting the warm, CaCl2-rich brine of the perennially ice-covered Lake Vanda, McMurdo Dry Valleys, Antarctica. Appl. Environ. Microbiol. 81, 1988–1995 (2015).
Kwon, M. et al. Niche specialization of bacteria in permanently ice-covered lakes of the McMurdo Dry Valleys, Antarctica. Environ. Microbiol. 19, 2258–2271 (2017).
Forte, E., Azzaro, M. & Guglielmin, M. Evidence of an unprecedented water erosion and supraglacial-fluvial sedimentation on an Antarctic glacier in the Holocene. Sci. Total Environ. 20, 20 (2022).
Doran, P. T. et al. Radiocarbon distribution and the effect of legacy in lakes of the McMurdo Dry Valleys, Antarctica. Limnol. Oceanogr. 59(3), 811–826. https://doi.org/10.4319/lo.2014.59.3.0811 (2014).
Saccò, M. et al. Salt to conserve: A review on the ecology and preservation of hypersaline ecosystems. Biol. Rev. 96, 2828–2850 (2021).
Ramoneda, J. et al. Importance of environmental factors over habitat connectivity in shaping bacterial communities in microbial mats and bacterioplankton in an Antarctic freshwater system. FEMS Microbiol. Ecol. 97, fiab044 (2021).
Saxton, M. A. et al. Sulfate reduction and methanogenesis in the hypersaline deep waters and sediments of a perennially ice-covered lake. Limnol. Oceanogr. 66, 1804–1818 (2021).
Frey, B. et al. Microbial diversity in European alpine permafrost and active layers. FEMS Microbiol. Ecol. 92, fiw018. https://doi.org/10.1093/femsec/fiw018 (2016).
Hu, W. et al. Characterization of the prokaryotic diversity through a stratigraphic permafrost core profile from the Qinghai-Tibet Plateau. Extremophiles 20, 337–349 (2016).
Alekseev, I., Zverev, A. & Abakumov, E. Microbial communities in permafrost soils of Larsemann Hills, Eastern Antarctica: Environmental controls and effect of human impact. Microorganisms 8(8), 1202 (2020).
Tian, R. et al. Small and mighty: Adaptation of superphylum Patescibacteria to groundwater environment drives their genome simplicity. Microbiome 8, 51 (2020).
Bowman, J. P., McCammon, S. A., Rea, S. M. & McMeekin, T. A. The microbial composition of three limnologically disparate hypersaline Antarctic lakes. FEMS Microbiol. Lett. 183, 81–88 (2000).
Aislabie, J. & Bowman J. P. “Archaeal Diversity in Antarctic Ecosystems.” Polar Microbiology: The Ecology, Biodiversity and Bioremediation Potential of Microorganisms in Extremely Cold Environments 31–59 (CRC Press, 2010).
Zhang, C. J. et al. Spatial and seasonal variation of methanogenic community in a river-bay system in South China. Appl. Microbiol. Biotechnol. 104, 4593–4603. https://doi.org/10.1007/s00253-020-10613-z (2020).
Bapteste, E., Brochier, C. & Boucher, Y. Higher-level classification of the archaea: Evolution of methanogenesis and methanogens. Archaea 1, 353–363 (2005).
Bowman, J. P. et al. Psychroflexus torquis gen. nov., sp. nov., a psychrophilic species from Antarctic sea ice, and reclassification of Flavobacterium gondwanense (Dobson et al. 1993) as Psychroflexus gondwanense gen. nov., comb. nov.. Microbiology 144, 1601–1609 (1998).
Donachie, S. P., Bowman, J. P. & Alam, M. Psychroflexus tropicus sp. Nov., an obligately halophilic Cytophaga-Flavobacterium-Bacteroides group bacterium from an Hawaiian hypersaline lake. Int. J. Syst. Evol. Microbiol. 54, 935–940 (2004).
Zhong, Z. P. et al. Psychroflexus salis sp. Nov. and Psychroflexus planctonicus sp. Nov., isolated from a salt lake. Int. J. Syst. Evol. Microbiol. 66, 125–131 (2016).
Chun, J., Kang, J. Y. & Jahng, K. Y. Psychroflexus salarius sp. Nov., isolated from Gomso salt pan. Int. J. Syst. Evol. Microbiol. 64, 3467–3472 (2014).
Yoon, J. H., Kang, S. J., Jung, Y. T. & Oh, T. K. Psychroflexus salinarum sp. Nov., isolated from a marine solar saltern. Int. J. Syst. Evol. Microbiol. 59, 2404–2407 (2009).
Buzzini, P., Turchetti, B. & Yurkov, A. Extremophilic yeasts: The toughest yeasts around?. Yeast 35, 487–497 (2018).
Coleine, C., Stajich, J. E. & Selbmann, L. Fungi are key players in extreme ecosystems. Trends Ecol. Evol. S0169–5347(22), 00025–00028 (2022).
Gonçalves, V. N. et al. Taxonomy, phylogeny and ecology of cultivable fungi present in seawater gradients across the Northern Antarctica Peninsula. Extremophiles 21, 1005–1015 (2017).
Ogaki, M. B. et al. Cultivable fungi present in deep-sea sediments of Antarctica: Taxonomy, diversity, and bioprospecting of bioactive compounds. Extremophiles 24, 227–238 (2020).
Wedin, M., Döring, H. & Gilenstam, G. Saprotrophy and lichenization as options for the same fungal species on different substrata: Environmental plasticity and fungal lifestyles in the Stictis-Conotrema complex. New Phytol. 164, 459–465 (2004).
Sterflinger, K. Black yeasts and meristematic fungi: Ecology, diversity and identification. In Biodiversity and Ecophysiology of Yeasts. The Yeast Handbook (eds Péter, G. & Rosa, C.) 501–514 (Springer, 2006).
Canini, F. et al. Growth forms and functional guilds distribution of soil Fungi in coastal versus inland sites of Victoria Land, Antarctica. Biology (Basel) 10, 320 (2021).
Vaniman, D. T. et al. Magnesium sulfate salts and the history of water on Mars. Nature 431, 663–665 (2004).
Gendrin, A. et al. Sulfates in martian layered terrains: The OMEGA/Mars Express view. Science 307, 1587–1591 (2005).
Carr, M. H. & Head, J. W. I. I. I. Geologic history of Mars. Earth Planet Sci. Lett. 294, 185–203 (2010).
Ojha, L. et al. Spectral evidence for hydrated salts in recurring slope lineae on Mars. Nat. Geosci. 8, 829–832 (2015).
Cragin, J. H., Gow, A. J. & Kovacs, A. Chemical fractionation of brine in the McMurdo Ice Shelf, Antarctica. CRREL Rep. 20, 83–86 (1983).
Frank, T. D. & Gui, Z. Cryogenic origin for brine in the subsurface of southern McMurdo Sound, Antarctica. Geology 38(7), 587–590. https://doi.org/10.1130/G30849.1 (2010).
Gardner, C. B. & Lyons, W. B. Modeled geochemical composition of cryogenically produced subglacial Brines, Antarctica. Antarct. Sci. 31(3), 165–166 (2019).
Lyons, W. B. et al. Halogen geochemistry of the McMurdo Dry Valleys lakes, Antarctica: Clues to the origin of solutes and lake evolution. Geochim. Cosmochim. Acta 69, 305–323 (2005).
Armienti, P. & Baroni, C. Cenozoic climatic change in Antarctica recorded by volcanic activity and landscape evolution. Geology 27(7), 617–620 (1999).
Di Nicola, L. et al. Multiple cosmogenic nuclides document complex Pleistocene exposure history of glacial drifts in Terra Nova Bay (northern Victoria Land, Antarctica). Quatern. Res. 71(1), 83–92 (2009).
Levy, R. et al. Late Neogene climate and glacial history of the Southern Victoria Land coast from integrated drill core, seismic and outcrop data. Glob. Planet. Change 80–81, 61–84 (2012).
Prebble, J. G., Raine, J. I., Barrett, P. J. & Hannah, M. J. Vegetation and climate from two Oligocene glacioeustatic sedimentary cycles (31 and 24 Ma) cored by the Cape Roberts Project, Victoria Land Basin, Antarctica. Palaeogeogr. Palaeoclimatol. Palaeoecol. 231, 41–57 (2006).
Tedersoo, L. et al. Shotgun metagenomes and multiple primer pair barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. Myco Keys 10, 1–43 (2015).
Andrews, S. FastQC: A quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc. (2010).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. https://doi.org/10.1038/s41587-019-0209-9 (2019).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. https://doi.org/10.1038/nmeth.3869 (2016).
Nilsson, R. H. et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res 47, D259–D264. https://doi.org/10.1093/nar/gky1022 (2019).
Acknowledgements
We want to thank to Programma Nazionale di Ricerca in Antartide (PNRA) for their incredible logistic support.
Funding
This article was funded by Programma nazionale di ricerca in Antartide (Grant no. PNRA18_00186-E).
Author information
Authors and Affiliations
Contributions
All the authors participated in the writing and in reviewing the papers. M.G. leaded the Project and the coordinated the research. M.G.; M.A.; S.P. made the field work; P.B., L.B., B.T., M.A., M.P., A.L., C.S. made all the microbiological analyses; while D.B. and M.R. made the chemical analyses; S.P. and M.G. made the glaciological analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guglielmin, M., Azzaro, M., Buzzini, P. et al. A possible unique ecosystem in the endoglacial hypersaline brines in Antarctica. Sci Rep 13, 177 (2023). https://doi.org/10.1038/s41598-022-27219-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-27219-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.