Preparation of diazoalkane complexes of iron(ii)†
Abstract
Diazoalkane complexes [Fe(η5-C5H5)(N2CAr1Ar2)(P–P)]BPh4 (1, 2) [P–P = Ph2PCH2CH2PPh2 (dppe) (1), Ph2PCH2CH2CH2PPh2 (dppp) (2); Ar1 = Ar2 = Ph (a); Ar1 = Ph, Ar2 = p-tolyl (b); Ar1Ar2 = C12H8 (c)] were prepared by allowing chloro compounds FeCl(η5-C5H5)(P–P) to react with diazoalkane in the presence of NaBPh4. Phosphine complex [Fe(η5-C5H5)(dppe)(κ1-PPh2CH2CH2PPh2)]BPh4 (3) was also prepared. Treatment of diazoalkane complexes 1, 2 with acrylonitrile afforded 3H-pyrazole derivatives 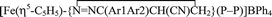 (4, 5), as well as a small amount of nitrile complex [Fe(η5-C5H5)(κ1-NCC(H)
(4, 5), as well as a small amount of nitrile complex [Fe(η5-C5H5)(κ1-NCC(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(P–P)]BPh4 (6). The complexes were characterised spectroscopically (IR, NMR) and by X-ray crystal structure determination of [Fe(η5-C5H5){N2C(C12H8)}(dppe)]BPh4 (1c).
CH2)(P–P)]BPh4 (6). The complexes were characterised spectroscopically (IR, NMR) and by X-ray crystal structure determination of [Fe(η5-C5H5){N2C(C12H8)}(dppe)]BPh4 (1c).


 Please wait while we load your content...
Please wait while we load your content...