Abstract
Seaweeds are a reserve of natural dyes (chlorophylls a, b and c), characterized by low cost and easy supply, without potential environmental load in terms of land subtraction, and also complying with the requirements of an efficient waste management policy. In particular, the brown seaweed Undaria pinnatifida is a species largely present in the Venice Lagoon area, and for it a removal strategy is actually mandatory. In this paper, we set-up an eco-protocol for the best extraction and preparation procedures of the pigment, with the aim of finding an easy and affordable method for chlorophyll c extraction, exploring at the same time the possibility of using these algae within local sustainable management integrated strategies, among which the possible use of chlorophylls as a dye source in dye sensitized solar cells (DSSCs) is investigated. Experimental results suggest that the developed protocols are useful to optimize the chlorophyll c extraction, as shown by optical absorption spectroscopy measurements. The DSSCs built with the chlorophyll extracted by the proposed eco-protocol exhibit solar energy conversion efficiencies are similar to those obtained following extraction protocols with larger environmental impacts.
Export citation and abstract BibTeX RIS
1. Introduction
Increasing concerns about the energy crisis, climate change, decreasing availability of fossil fuels and environmental issues are increasingly pushing the research on sustainable and renewable energy resources [1]. Among the current photovoltaic technologies, inorganic solid-state junction devices that are usually made of silicon are still dominating the photovoltaic field and industry because of the good compromise between cost and efficiency [2]. Nevertheless, the dye sensitized solar cell DSSCs [3] have drawn great attention in the scientific community for their ease of fabrication, low cost and competitiveness with different photovoltaic systems based on p–n junctions.
The operation mechanism of a DSSC resembles that of the natural photosynthesis, involving light-energy absorption and charge separation, a process that suggested the potential use of the photosynthetic pigments for fabricating DSSCs [4]. Metal-organic dyes, mostly ruthenium and osmium polypyridil complexes, have been used as efficient sensitizers [5–7]. However, the preparation routes for metal complexes are often based on multistep reactions involving complex and expensive chromatographic purification procedures [1]. On the other hand, natural pigments have been considered as promising alternative sensitizer dyes because of their simple preparation technique, low cost, complete biodegradation, availability, purity grade, water solubility, environmental friendliness, and, most importantly, significant reduction of noble metal and chemical synthesis cost [2, 8, 9].
Plant pigmentation results from the electronic structure of pigments reacting with sun light, pigments characterized by a maximum wavelength (λmax) of their respective selective absorption [10]. The performance of a natural dye sensitizer in DSSC is evaluated by measuring open circuit voltage (Voc), short circuit current density (Jsc), fill factor (FF), and energy conversion efficiency (η), whereas the dye absorption spectrum and the anchorage to the surface of the semiconductor TiO2 are also important parameters for determining DSSC efficiency [2, 9].
Currently, the main part of chlorophylls extracted from terrestrial plants and marine algae finds application as a natural dye in the food industries, or as phytotherapic substances in the pharmaceutical and cosmetic fields. As mentioned before, several natural dyes have also been used as sensitizers in DSSCs [1, 2] exhibiting high energy conversion efficiency, such as chlorophyll [11–14], carotenoid [15–17], anthocyanin [1, 18–20], flavonoid [2, 10], and tannin [21]. Chlorophylls a and b and their derivatives are the most commonly used as sensitizers in DSSCs because of their absorbance in the blue and red light regions. The most efficient is a derivative of chlorophyll a (methyl trans-32-carboxy-pyropheophorbide) [10]. The absorbance spectrum of chlorophyll b exhibits a characteristic feature in the blue region, with a redshift when compared to chlorophyll a [22].
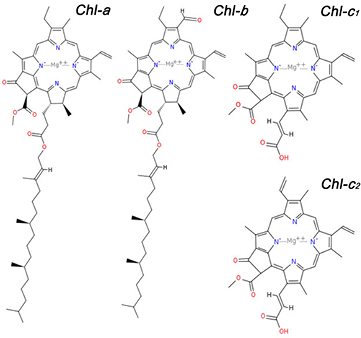
Chlorophyll a does not adsorb efficiently on TiO2 due to the weak interaction of its ester and keto carbonyl groups with the hydrophilic oxide surface. Chlorophylls c (Chls) are generally present as a mixture of Chls-c1 and Chls-c2 in brown algae. They possess a terminal carboxyl group, which is connected to the porphyrin macrocycle through a conjugated double bond (figure 1). Thanks to this anchoring group, they can bind strongly to TiO2 and can ensure efficient electron injection into the TiO2 conducting band, preventing the gradual leaching by the electrolyte.
Figure 1. General structure of chlorophyll types contained in macroalgae.
Download figure:
Standard image High-resolution imageNatural dyes are obtained with or without chemical treatments. Terrestrial plants are the main natural stock of dyes that can be easily extracted from fruit, leaves and flowers. However, seaweeds are one of the most abundant photosynthetic species containing c-type chlorophylls, and as a matter of fact seaweeds are presently an underexploited resource [23–25].
In this work, we focused our investigation on brown seaweed U. pinnatifida, in order to: (i) find an easy, affordable and eco-friendly method for chlorophyll c extraction; (ii) integrate the possible use of this alga within sustainable management integrated strategies in lagoon areas.
Venice Lagoon is the largest Mediterranean transitional environment, and a hot-spot for the introduction of new species, including the Japanese brown seaweed U. pinnatifida. This alga, established in Venice Lagoon since 1993, with fronds of about 1–2 m, produces high biomass quantities, displacing other native species and disturbing the navigation. An eradication intervention would not be feasible, mainly because the species is present with stable populations and thrives in sites rich in anthropic activities. On the other hand, an exploitation of this brown seaweed would result in turning a waste product into a valuable resource.
2. Material and methods
2.1. Chlorophyll extraction
Samples of brown seaweed U. pinnatifida were collected in Venice Lagoon (45° 26' 00.8'' N; 12° 18' 40.4'' E) during Spring 2013. The samples were washed in running water and stored at −20 °C until chlorophyll extraction. Defrosted algae were rinsed twice with tap water and distilled water, blotted dry and roughly cut into small pieces. Sample fragments (3 grams) were then placed in 15 ml of extraction buffer and the mixture was sonicated 15–20 min to facilitate chlorophyll release. Extraction was then carried out overnight at 4 °C. Solid residues were removed by centrifugation (12 000 g for 10 min at 4 °C).
Extraction buffer was prepared using different percentages of acetone diluted in water. Six different acetone percentages were tested: 100%, 80%, 70%, 60%, 50% and 40%.
Chlorophylls present in the supernatant were measured using an UV-visible spectrophotometer Agilent 8543, with 100 μl of each sample diluted with assay solvent to obtain up to 1 ml of final mixture to acetone 90%. The Ritchie algorithms [26] were used to determine chlorophyll concentrations:

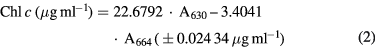
where the Chl type concentrations are obtained by a combination of absorbance values taken at λ = 630 nm (A630) and λ = 664 nm (A664), each multiplied for a specific absorbance coefficient (in μg ml−1 cm units) for absorbance values expressed as cm−1. All replicates and the procedures were performed protecting the sample from light to ensure pigment stability.
2.2. DSSCs fabrication
2.2.1. Preparation of TiO2 paste and nanocrystalline electrodes.
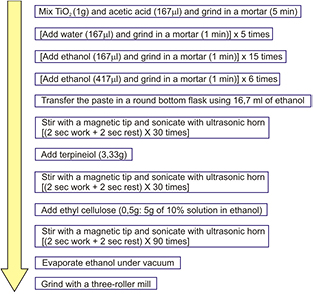
The procedure for TiO2 paste fabrication is depicted in figure 2. A fluorine-doped tin oxidecoated glass slide (FTO; Sigma-Aldrich, 50 × 50 × 2.2 mm3, sheet resistance 7 Ω cm−2) was used as the current collector. It was cleaned in a detergent solution using an ultrasonic bath for 15 min, and then rinsed with water and ethanol. The cleaned working electrode was coated with the TiO2 blocking layer. The deposition was performed by the spray pyrolysis technique, using a solution 0.16 M diisopropoxytitanium-bis(acetylacetonate) (TAA) and 0.4 M acetylacetone (ACAC) in ethanol on a conductive glass sheet at 300 °C. The electrodes coated with the TiO2 blocking layer were gradually heated in air at 125 °C for 15 min, at 450 °C for 10 min and finally at 500 °C for 20 min, with 10 °C min−1 temperature ramp.
Figure 2. Scheme of paste fabrication from nanocrystalline TiO2 powder 10 nm. All steps were performed at room temperature.
Download figure:
Standard image High-resolution imageA layer of paste was coated on the FTO glass plates using the doctor blade technique, kept in a clean box for 3 min with ethanol so that the paste can relax to reduce the surface irregularity and then dried for 10 min at room temperature. The electrodes coated with the TiO2 pastes were gradually heated in air at 125 °C for 15 min, at 325 °C for 10 min, at 375 °C for 10 min, at 450 °C for 15 min and finally at 500 °C for 30 min, with 10 °C min−1 temperature ramp. After sintering, the thickness of the TiO2 electrodes was measured with a profilometer.
2.2.2. DSSC assembling.
The TiO2 film was treated with 50 mm in a TiCl4 solution, soaking each electrode in 50 mM TiCl4 aqueous solution at 70 °C for 30 min, then rinsed with water and ethanol and sintered at 500 °C for 30 min. At 50 °C in the cooling, the TiO2 electrode was immersed into a 50 μM Chl solution in 60% acetone, previously concentrated via rotavapor, and placed in a dark container at room temperature for 12 h to complete the sensitizer uptake.
To prepare the counter-electrode, a hole was drilled in the FTO glass, then washed with water and with a 0.1 M HCl solution in ethanol, and ultrasonically cleaned in acetone for 10 min. After removing residual organic contaminants by heating in air for 15 min at 400 °C, the Pt catalyst was deposited on the FTO glass by spray pyrolysis with a H2PtCl6 solution (0.5 mg Pt in 1 ml ethanol), then repeating the heat treatment at 400 °C for 20 min.
The dye-covered TiO2 electrode and the Pt counter-electrode were assembled into a sandwich type cell and sealed with a hot-melt, 35 μm thick gasket made of the ionomer Bynel 4702 (Du-pont). The aperture of the Surlyn frame is 1 mm larger than that of the TiO2 area, and its width is 2 mm. A drop of the electrolyte, a solution 0.6 M 1,2-dimethyl-3-propylimidazolium iodide (DMPII), 0.1 M lithium iodide, 0.05 M iodine and 0.5 M 4-tert-butylpyridine in the mixture of acetonitrile and valeronitrile (volume ratio 85:15), was put on the hole in the back contact of the counter-electrode. The electrolyte was introduced into the cell via vacuum backfilling. The cell was placed in a small vacuum chamber to remove inside air. By exposing it again to ambient pressure, the electrolyte was driven into the cell. Finally, the hole was sealed using a hot-smelt ionomer film (Bynel 4702, 35 μm thick, Du-Pont) and a cover glass (0.1 mm thick).
The system used for measuring the power delivered by the cell was made of an ABET Technologies solar simulator as the light source, to approximate the 1.0 air mass solar spectrum, and a digital multimeter (Tektronix Keithley 2410) directly connected to the cell. The power-against-voltage P–V curve was obtained by simultaneously measuring the flowing current I and the cell voltage V (P = V ⋅ I).
3. Results and discussion
3.1. Chlorophyll extraction
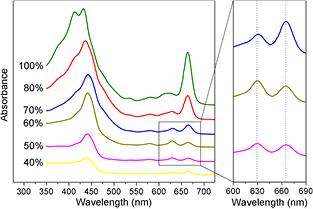
Optical absorption spectra (figure 3) exhibit different features, pointing out that the type of chlorophyll extracted varies with the different percentages of acetone used. In particular, the characteristic Chl-c peak at 630 nm is mostly evident for 60% acetone dilution, while the presence of the peak at 665 nm indicates the extraction of the Chl-a using 100% of acetone.
Figure 3. Optical absorption spectra of samples at different percentages of acetone; the right panel evidences the behavior exhibited by the characteristic Chl-c and Chl-a Q-bands peaked at 630 and 664 nm, respectively.
Download figure:
Standard image High-resolution imageBased on the Chl concentration values estimated by means of Ritchie's algorithms, it is possible to determine the extraction yield per gram of fresh alga using different acetone percentage solutions: the best results are 654 μg g−1 (concentration of 12.04 μg ml−1) with 100% acetone solution and 79 μg g−1 (concentration of 1.65 μg ml−1) with 60% acetone solution for Chl-a and Chl-c, respectively. The 100% of acetone buffer extraction gives one of the highest absolute values of extracted Chl-c (1.38 μg ml−1) but at the same time exhibits the lowest purity, with a Chl-c/Chl-a ratio of 0.1. On the other hand, extraction performed with the 50–60% of acetone buffer gives the best results for both Chl-c amount and purity, with a Chl-c/Chl-a ratio of 1.8 (table 1). These findings are ascribed to the long hydrophobic hydrocarbon tail of the Chl-a molecule, attached to a porphyrin macrocycle, and therefore more soluble in non-polar solvent (i.e. acetone). Chl-c is more hydrophilic than Chl-a and Chl-b, since it lacks the hydrophobic tail typical of those chlorophylls (see figure 1). According to Wright et al [27], the different polarity of chlorophylls can be used as a separation principle: as water increases the polarity of the solvent, thus preventing chlorophyll from being released by the membranes, this allows the relative amount of Chl-c to be increased in the total extracted pigments.
Table 1. Chlorophyll concentration values (μg ml−1) as estimated by Ritchie's algorithms for the 90% acetone diluted solutions, with chlorophyll extraction yields per gram of algae specimen (μg g−1) and relative Chl-c/Chl-a ratio for extraction buffers prepared with different acetone percentage solutions.
| Acetone (%) | Chl concentration | Chl extraction yield | |||
|---|---|---|---|---|---|
| Chl-a (μg ml−1) | Chl-c (μg ml−1) | Chl-a (μg g−1) | Chl-c (μg g−1) | Chl-c/Chl-a ratio | |
| 40 | 0.43 | 0.45 | 24 | 22 | 0.9 |
| 50 | 0.70 | 1.15 | 32 | 57 | 1.8 |
| 60 | 0.92 | 1.65 | 44 | 79 | 1.8 |
| 70 | 1.51 | 1.67 | 70 | 76 | 1.1 |
| 80 | 3.97 | 1.40 | 196 | 69 | 0.4 |
| 100 | 12.04 | 1.38 | 654 | 75 | 0.1 |
The variation of the chlorophyll concentrations for different acetone percentages and the ratio curve Chl-c/Chl-a are shown in figure 4.
Figure 4. Chlorophyll extraction yields (Chl-a and Chl-c) and ratio-curve (Chl-c/Chl-a) for different percentages of acetone, with error bars. Data are reported in table 1.
Download figure:
Standard image High-resolution image3.2. DSSC performance
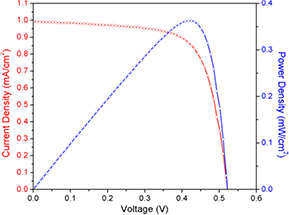
The recorded P–V curve (figure 5) presents the following parameters: Voc = 0.52 V, Jsc = 0.99 mA cm−2 and efficiency 0.36%, with FF = 0.71. As reported in table 2, the detected values are comparable with those for seaweeds' chlorophyll dye for DSSCs from the literature [23, 28].
Figure 5. P–V curve for U. pinnatifida.
Download figure:
Standard image High-resolution imageTable 2. DSSC parameters obtained from seaweeds.
| Jsc (mA cm−2) | Voc (V) | Pmax (mW cm−2) | FF | η (%) | |
|---|---|---|---|---|---|
| Calogero et al [23] | 0.8 | 0.36 | 0.178 | 0.69 | 0.178 |
| Taya et al [28] | 0.397 | 0.559 | 0.1 | 0.44 | 0.1 |
| This work | 0.99 | 0.52 | 0.36 | 0.71 | 0.36 |
Compared to the literature, the use of 4-ter-butyl-pyridine additive in the electrolyte does not reduce the current density, and boosts the Voc value. Furthermore, the shape of the P–V curve depends on a good match with the employed electrolyte. With the aim of improving the current density, longer soaking time and concentrated solutions should be used, due to possible effects of dye aggregation [11] as the origin of low photocurrent and conversion efficiency [29]. In general, two kinds of aggregation can occur: a stacked face-to-face Chl π-aggregation (sandwich-type, H- aggregate), and a side-by-side chlorine π-aggregation (J-aggregate), with a decreasing of the sensitization activity. Chl-a not having free carboxylic group to anchor to the TiO2 layer remains on the photoanode aggregated to the Chl-c by J or H aggregation, thus not promoting dye aggregation. Further work on the use of fatty acid with long alkyl chain or rigid structure to inhibit this aggregation is in progress.
The co-existence of water and acetic acid is important in the preparation of TiO2 paste. During these experiments, another combination with acetyl acetone, polyethylene glycol, triton X-100 and so on was also tested. Without water and/or acetic acid, however, the porous-TiO2 layers over 10 mm thickness are mechanically unstable and do not preserve the structure after sintering due to large cracks and peeling-up from substrate.
4. Conclusions
A preliminary investigation was presented for the development of an eco-protocol for Chl-c extraction, using the brown seaweed U. pinnatifida from Venice Lagoon as the natural resource, in order to match integrated management strategies for the employment of local seaweed.
The presented eco-protocol has been demonstrated to be suitable to improve the ratio between Chl-c and Chl-a (this last not adsorbing efficiently on TiO2 due to the weak interaction of its ester and keto carbonyl groups with the hydrophilic oxide surface). This protocol allowed us to obtain a good amount of Chl-c (79 μg g−1) using an easy and fast procedure, characterized by a low environmental impact. Dye sensitizer solar cells built-up with the extracted pigments exhibited efficiency (0.36%) and fill factor (0.71) values comparable to data reported in the literature for U. pinnatifida seaweed.
Work is in progress to improve the photovoltaic conversion of DSSCs by acting on the dye sensitizer performance, in particular, by suppressing the aggregation of Chl-a molecules on Chl-c, as well as by modifying the molecular structure of the extracted chlorophyll.
Acknowledgments
We thank Dr G Calogero, CNR-IPCF of Messina (Italy), for useful advice and helpful discussions.