Long-term data prove useful to keep track of non-indigenous seaweed fate
- 1Institute for Water Research (IRSA)-CNR, Taranto, Italy
- 2Department of Environmental Sciences, Informatics and Statistics, University of Venice Ca’ Foscari, Venice, Italy
- 3Department of Chemical, Pharmaceutical and Agricultural Sciences, University of Ferrara, Ferrara, Italy
- 4Department of Biosciences, Biotechnologies, and Environment, University of Bari, Bari, Italy
The Mar Piccolo of Taranto (southern Italy, Mediterranean Sea), a site of the European LTER network, is a transitional water system, where a century-old intensive mussel farming activity has been carried out, together with an intense import-export business of bivalve mollusks. Previous studies showed that this basin is third for NIS seaweed introduction in the Mediterranean Sea, after the Thau Lagoon and the Venice Lagoon. The present paper deals with the results of 11-year monitoring activity on non-indigenous species (NIS) of seaweeds, which was performed in the Mar Piccolo. In the studied period (2011–2021), two different time frames (i.e., 2011–2015 and 2016–2021) were considered, since they were based on a different number of sampling sites. To investigate spatial and temporal differences in the seaweed assemblage, a multivariate analysis was performed considering the NIS and the most important native species in terms of temporal occurrence. Fourteen NIS were recorded in total in the Mar Piccolo of Taranto during this period, with variable abundances among sites and years: nine species in the first time period, and thirteen species in the second one. Caulerpa cylindracea, recorded with negligible biomass in the first period, was absent in the second-period samplings. Molecular analyses confirmed the taxonomy of three species (i.e., Grateloupia minima, Neopyropia koreana, and Polysiphonia morrowii), previously identified only through morphological features. The most abundant species was Hypnea corona, which almost doubled its biomass in the second time period compared to the first one. Three species (i.e., Caulacanthus okamurae, G. minima, and P. morrowii) increased their biomass by an order of magnitude in the second time period. No significant differences were found over years. Site 1 resulted in significant differences among the sites and different seasonal pattern occurred among the investigated sites. No significant long-term changes occurred in the seaweed assemblages, suggesting the absence of strong disturbances due to the settlement of NIS.
1 Introduction
It is well known that long-term ecosystem observations and measurements are an invaluable means to understanding the causes and consequences of perturbations occurring (Turner et al., 2003). For this reason, Long Term Ecological Research (LTER) networks have been established over the years both at national and international levels, starting from the first United States network that began its activities in 1980 (Callahan, 1984). Nowadays, the LTER series have become a starting point to assess and interpret the effects of climate change and other anthropogenic pressures (Pugnetti et al., 2013; Zingone et al., 2019). In particular, the importance of LTER datasets for biodiversity studies has been underlined (Compagnoni et al., 2020), and the United Nations Convention on Biological Diversity considers them recommended indicators (Zilioli et al., 2019).
Since 2011 the Mar Piccolo of Taranto has been part of the Italian and European LTER network (https://deims.org/ac3f674d-2922-47f6-b1d8-2c91daa81ce1), mainly due to the presence of historical data sets on chemical-physical variables and benthic macrophyte biodiversity (Petrocelli et al., 2021). It is a transitional water system, where intensive mussels farming activity has been carried out for approximately 100 years, as well as a strong import site for bivalve mollusks. For this reason, it is highly exposed to the introduction of non-indigenous species (NIS) (Newton et al., 2014), in particular, seaweeds (Mineur et al., 2014; Wolf et al., 2018). According to Copp et al. (2007), the frequency and success rate of NIS introduction are closely related to propagule pressure too. In Australia, after a boom-population, the invasive Codium fragile heavily declined up to densities lower than 2 thalli/m2, most likely due to the presence of very few propagules with limited viability (Trowbridge et al., 2016). Conversely, in a newly-built marina in Brittany (France) Undaria pinnatifida (Harvey) Suringar reached densities of up to 50 specimens/m2 in 2 years, due to a continuous propagule supply from populations settled on adjacent rocky shores (Salamon et al., 2020). Climate conditions are another important factor that affects the establishment of NIS, becoming more limiting in transitional waters. The tropical Grateloupia yinggehaiensis H.W. Wang et R.X. Luan had a considerable spread in the industrial area of Porto Marghera (northern Adriatic, Mediterranean Sea), due to the thermal pollution caused by a thermoelectric power plant operating in the area (Wolf et al., 2014).
In the Mar Piccolo of Taranto, the regular and longtime LTER data collection has allowed a continuous update of the basin phytobenthic biodiversity, the prompt detection of newly introduced NIS, the assessment of NIS fate, and the detection of the most important factors for either their success or decline (Petrocelli et al., 2019). For example, two seaweed species have been observed only temporarily and disappeared without establishing in the basin. In June 2012, for the first time and no longer after that date, very few thalli of Ascophyllum nodosum (Linnaeus) Le Jolis were observed on sparse pebbles in the Mar Piccolo. This species was first reported in the Mediterranean in 2009, after the casual finding of very few floating specimens in the Mar Grande basin, near a mollusk import-export center (Petrocelli and Cecere, 2010). This led to the hypothesis that the introduction vector for A. nodosum in Taranto seas was oyster imports from France. Ascophyllum nodosum thalli were most likely used to cover and keep the mollusks fresh during transportation (Verlaque et al., 2007), and then dumped in the seawater. Since this species is native to the cold-temperate waters of the North Atlantic, with a temperature tolerance not higher than 25°C (Keser et al., 2005), and the temperature of Mar Piccolo seawater is often higher than 27°C (Petrocelli et al., 2020a), this conceivably prevented the establishment of A. nodosum (Petrocelli et al., 2013). Undaria pinnatifida, a cold-temperate species native to the Pacific Japanese seawaters, underwent a similar fate in the Mar Piccolo. After first detection in 1998, this species showed a short initial period of increasing settlement, followed by a quick decline and its final disappearance over 11 years (Cecere et al., 2016). Laboratory and field observations in the native area have suggested an optimum temperature of 19°C for U. pinnatifida reproduction and survival (Akiyama and Kurogi, 1982; Sanderson, 1990; Watanabe et al., 2014). The temperature extremes recorded in Mar Piccolo seawater, both in the coldest week and in the warmest one in the last year of U. pinnatifida flourishing growth, were far from the optimum. This most likely contributed to U. pinnatifida disappearing, even though the additional and combined influence of low salinity and heavy metal contamination could not be excluded (Cecere et al., 2016).
The presence of NIS seaweeds is often overlooked due to their possible morphological similarity with native species. In this case, molecular analyses through the DNA barcoding method have proved to be fundamental in discriminating cryptic introduced species (Zucarello et al., 2002; Saunders, 2009; Montes et al., 2016; 2017; Piñeiro-Corbeira et al., 2020). In the Mar Piccolo, DNA barcoding analyses confirmed the taxonomic identity of several species previously identified through classical morphological methods. Agardhiella subulata was initially identified through a morphological analysis (Perrone and Cecere, 1994). In 2011, after a new introduction event, its identification was confirmed by the rbcL gene analysis (Cecere et al., 2011a). In December 2000, specimens of Hypnea cornuta (Kützing) J. Agardh were collected in the Mar Piccolo for the first time. The species identification was based on anatomical features and the presence of stellate propagules (Cecere et al., 2004), as well as confirmed by molecular analysis (Yamagishi et al., 2003). Since the taxonomic identity of this taxon became less defined over time, recently a molecular study was focused on the H. cornuta complex, detecting three different clades inside this group (Jesus et al., 2019). The Taranto specimens, together with those from Western Australia, New Zealand, and Japan were ascribed to a generic Clade#3, which was then named Hypnea corona Huisman and Petrocelli (Huisman et al., 2021). Due to the presence of ambiguous morphological features, some specimens of a rhodophycean seaweed collected in the Mar Piccolo in 2010, were doubtfully identified as Grateloupia cf. filicina (J.V. Lamouroux) C. Agardh, a species exclusively distributed in the Mediterranean Sea (De Clerck et al., 2005; Wilkes et al., 2005). Molecular analyses, based on the rbcL gene, were therefore carried out on these specimens from Mar Piccolo and also on specimens from the French Thau Lagoon collected in 1998 and tagged as Grateloupia sp. This molecular survey identified all the samples as G. minima P. and H. Crouan, described in 1867 from the Atlantic Ocean, allowing its first report as a NIS both in the Mar Piccolo and in the Mediterranean Sea (Cecere et al., 2011b). The taxonomic identity of Grateloupia turuturu specimens collected in the Mar Piccolo and the Venice Lagoon in 2007, was confirmed through DNA analysis in recent years (Cecere et al., 2011c). A turf-forming rhodophycean species, observed for the first time in the Mar Piccolo in 2012, was identified based on the morphological analysis as Caulacanthus ustulatus (Turner) Kützing (Rhodophyta, Gigartinales), a taxon commonly distributed in the Mediterranean Sea. Some specimens from the French Mediterranean coast were successively ascribed to the NIS taxon Caulacanthus okamurae (Verlaque et al., 2015). Therefore, rbcL gene molecular analyses were carried out on C. ustulatus specimens collected both in the Mar Piccolo and in the Venice Lagoon, confirming the presence of the non-indigenous taxon also in these two Mediterranean hot spots (Petrocelli et al., 2020b).
With this background and exploiting the long-term data potential, this paper aims to take stock of the NIS seaweed situation in the Mar Piccolo of Taranto, also through the help of molecular methods. Continuous introductions have been recorded over time, with the different species displaying many adaptive strategies. Therefore, several years after their first detection, they show different distribution pathways and fates. Based on their growth and reproduction requirements, if known, their further development has been hypothesized too. Climate change, and in particular heat waves, are becoming well-known phenomena; thus, the less adaptive species will most likely disappear from the basin. Finally, the DNA barcoding method has further proved to be a useful means for cryptic introduced species issues, otherwise doubtfully identified with classical approaches.
2 Materials and methods
This research was carried out in the Mar Piccolo of Taranto (40°28′46″ N, 17°13′41″ E) (southern Italy, Mediterranean Sea). It has a somewhat elliptical shape and is divided into two sub-basins, the First Inlet and the Second Inlet (Figure 1). The Mar Piccolo is a transitional water system, characterized by the input of brackish water, at a mean temperature of 18°C and a salinity range of 2.3–4.7, coming from 34 submarine springs, locally named “citri,” variously distributed in the two Inlets. In the basin, seawater temperature ranges between 7.5°C and 32.3°C, while salinity ranges between 33.0 and 37.8.
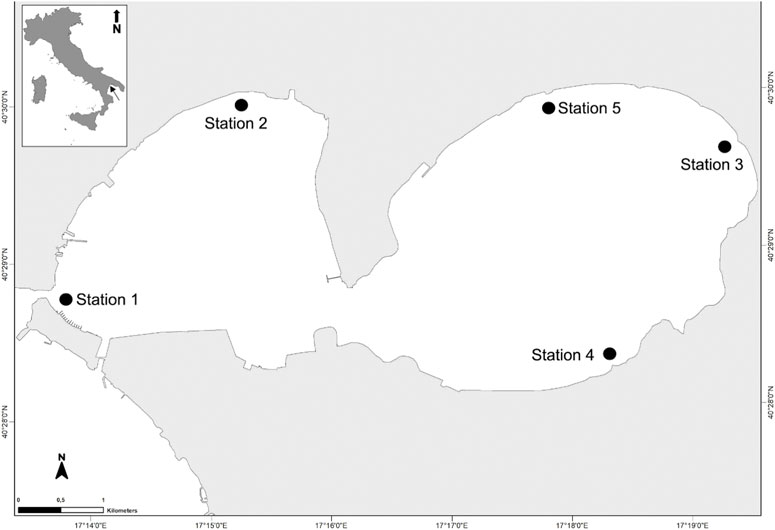
FIGURE 1. Map of the Mar Piccolo of Taranto and location of sampling sites. The arrow in the frame indicates the location of Taranto in Italy.
During the 11-year study, two time periods were considered due to a different number of investigated sites. From 2011 to 2015, the sampling sites were four, two in the First Inlet and two in the Second Inlet (Figure 1). In 2016, a fifth site, located in the Second Inlet, was added (Figure 1).
Seasonal manual harvests were carried out at a maximum depth of 50 cm. For each site, three 50 cm × 50 cm quadrats were collected by scraping the surface within the quadrats and picking all the seaweeds. Seaweeds were put in plastic bags identified by an encoded box for each replicate and brought to the laboratory, where they were stored in plastic nets and dipped in concrete tanks filled with seawater. Harvested samples were individually sorted to separate single species. After sorting, taxonomic identification of each taxon was performed through stereomicroscopes (LEICA MZ 12 Stereoscope, Leica Microsystems GmbH, Wetzlar, Germany) and light microscopes (LEICA DMR Trinocular Microscope, Leica Microsystems GmbH, Wetzlar, Germany). Successively, biomass (drained wet weight) was measured by means of a double-digit analytical balance (Sartorius L2200P, Sartorius Lab Instruments GmbH, Göttingen, Germany).
2.1 Molecular analyses
Since the identification was considered dubious for some specimens, a small part of the thallus was carefully cleaned from epiphytes and debris and dried in silica gel (J.T. Baker, Deventer, Holland) for molecular analyses.
Silica-dried fragments of thalli were ground in a mortar with quartz sand (Honeywell Fluka, Charlotte, NC, United States), and total genomic DNA was extracted with the Genomic DNA Purification Kit (Thermo Fisher Scientific, Waltham, Massachusetts, United States) following the manufacturer’s recommendations. Since the dubious specimens belonged to the phylum Rhodophyta, the 5P portion of the rbcL gene (rbcL-5P) was selected to identify them following the DNA barcoding method.
The rbcL-5P fragment (about 700 bp) was amplified using the primer pairs F57-R753 (Freshwater and Rueness, 1994) and the PCR conditions reported by Wolf et al. (2011). The obtained amplicons were verified by agarose gel electrophoresis and purified with ExoSAP-IT (Thermo Fisher Scientific, Waltham, Massachusetts, United States) before sequencing. Sequencing was performed at the Eurofins Genomics Sequencing Service (Eurofins Genomics GmbH, Ebersberg, Germany) with the same primer pairs used for amplification. Final consensus sequences were assembled with the help of the GeneStudio program (http://genestudio.com/) and then compared with the sequences available in the INSDC (International Nucleotide Sequence Database Collaboration) archives using the BLAST tool (Altschul et al., 1990). The newly obtained sequences were also deposited in the INSDC archives through the GenBank platform BankIt.
2.2 Multivariate analysis
A multivariate analysis was carried out to explore spatial and temporal differences in the macroalgae assemblages. Three sites (1, 3, 4) were chosen, since they were sampled along the entire time series and represent different zones in the First and Second Inlet of the Mar Piccolo (Figure 1). In this way the sampling design resulted less unbalanced. The characteristic species of the assemblage were selected according to their frequency of occurrence (FO%, expressed in percentage) during the entire period, selecting the species with a FO% > 5% and maintaining all NIS in the analysis (Supplementary Table S1). A total of 37 species were selected and the biomass data (g m-2) were four-root transformed to balance the contribution of rare and very abundant species. Multivariate analyses were conducted by means of a Bray-Curtis similarity calculated on a “stations × species” matrix (377 × 37).
The null hypothesis of no spatial variation or temporal differences in seaweed assemblage structure was investigated by adopting a multifactorial model tested by the Permutational Multivariate Analysis of Variance (Anderson et al., 2008). Data collected from each site were considered independent because they were carried out in random positions within each site, and thus, the exchangeability of the observations under the null hypothesis was assumed, fulfilling the requirements of hypothesis testing with permutation methods (Anderson, 2001). Year (fixed with 11 levels), Season (fixed with 4 levels), and Site (random with 3 levels) were tested as orthogonal factors in the PERMANOVA test, with p-values calculated through 9,999 permutations using the “Permutation of residuals under a reduced model” as permutation method. In the model, “Year × Site” and “Season × Site” interactions were tested. When the PERMANOVA test result was significant (p < 0.05), a post hoc PAIRWISE t-test was carried out to evaluate differences between the levels of each factor and their interactions, calculating Monte Carlo p-values (Anderson et al., 2008). Data were plotted using both unconstrained ordinations, the nonmetric multidimensional scaling (nMDS, Clarke and Warwick, 2001), and Principal Coordinate Analysis, PCoA, Gower, 1966). In both ordination methods, the sites were plotted together with the species mainly correlated to the first two axes in the PCoA using Spearman’s correlation coefficient (rs). In addition, the distances among centroids were explored for significant complex interactions between factors using both ordination methods to visualize complex spatio-temporal patterns (Guerra-Castro et al., 2016).
The contribution of species to the differences among sites was explored through the Similarity Percentage analysis (SIMPER; Clarke, 1993; Clarke and Warwick, 2001). All multivariate analyses were carried out by means of PRIMER v.6+PERMANOVA (Primer-E Ltd., Plymouth, United Kingdom).
3 Results
3.1 NIS species
In the studied period (2011–2021), fourteen NIS were recorded in the Mar Piccolo of Taranto. In the 2011–2015 time frame, nine species were recorded in four sites. In the 2016–2021 time frame, thirteen species were found in five sites. Eight species were recorded throughout the study period, one species was present only from 2011 to 2015, and five species were present only from 2016 to 2021 (Table 1).
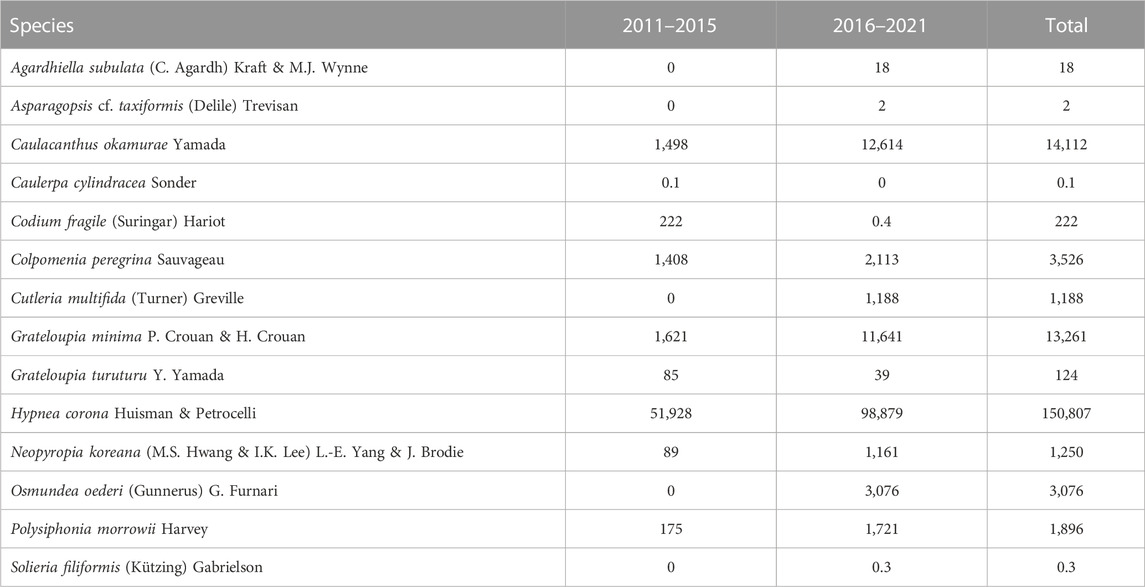
TABLE 1. List of NIS and their total abundances (gwwt m−2) recorded in the Mar Piccolo of Taranto in the study period 2011–2021.
Figure 2 reports the biomass trend of NIS species in the studied period. Due to their very low biomass values, A. subulata, Asparagopsis taxiformis, Caulerpa cylindracea, and Solieria filiformis were not plotted. In both time frames, the highest total biomass values were reached by H. corona (Table 1). It was observed only in the Second Inlet, from spring to autumn with ups and downs throughout the study period. From 2011 to 2015, it was present at Site 3 and Site 4, while from 2016 to 2021 also at Site 5. For each year, summer was the season of maximum abundance. For each year and in all the seasons, Site 3 collected quantities were higher than those of Site 4, while starting from 2016, H. corona abundance at Site 5 sometimes exceeded that at Site 3. The highest yearly total biomass value was measured in 2018 and corresponded to 28.8 × 103 gwwt m−2.
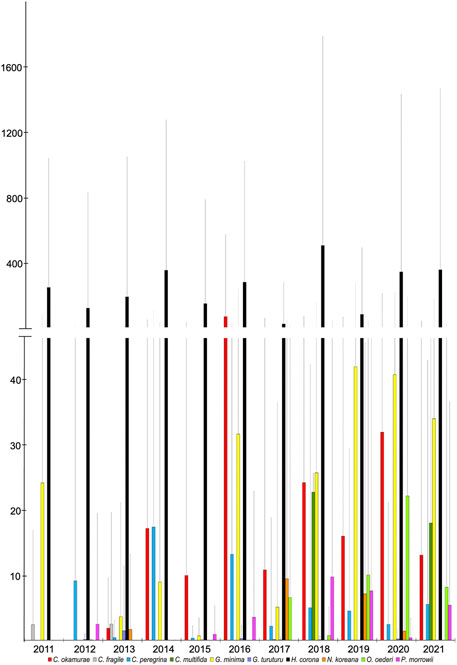
FIGURE 2. Abundances (mean total value + s.d.) of NIS collected in the Mar Piccolo for each year in the study period 2011–2021.
In the summer of 2012, for the first time and with very few specimens, C. okamurae was recorded at Site 4; it was settled on basal parts of Chondracanthus acicularis (Mertens ex Turner) Kützing. Since then, it was observed every year and in every season, mainly at Site 4, but also occasionally with small quantities at the other sites (except for Site 2), reaching the peak in spring 2016 (i.e., 6.4 × 103 gwwt m−2). From 2017, a considerable decrease was observed, with ups and downs until autumn 2021.
Grateloupia minima was collected for the first time in 2011 at Site 4. Successively, it was recorded every year mainly at Site 4, and since 2016 also at Site 5, where it became one of the most luxuriant species in the last 3 years. The highest total yearly biomass value, corresponding to 2.7 × 103 gwwt m−2, was reached in 2019.
Colpomenia peregrina was observed for the first time in the winter of 2012 at Site 1. Since then, it was observed every year only at the same site, with the following seasonal occurrence: always in spring, several times in winter (except in 2014, 2015, and 2017), and fewer occurrences in autumn (i.e., 2014, 2015, and 2020). The yearly biomass peak was reached in 2014, with 9.0 × 102 gwwt m−2. Considering the whole study period, C. peregrina biomass showed ups and downs in the first time frame and reached a certain uniformity in the second time frame, with values considerably lower compared to the peak.
The presence of Cutleria multifida was detected for the first time in 2017, even though it was collected with measurable biomass only in the winters of 2018 and 2021 at Site 1. The highest yearly total biomass value was reached in 2021 and corresponded to 1.2 × 103 gwwt m−2. Concerning Neopyropia koreana, a very low biomass was recorded for the first time in winter 2012 at Site 4. Successively, this species was collected always in winter only at Site 1 and with highly variable biomass values. The highest total yearly value was recorded in 2017 and corresponded to 6.1 × 102 gwwt m−2. Polysiphonia morrowii was found for the first time in the winter of 2012 at Site 1; since then, starting from 2014, it was recorded every year at the same site, and from 2015 also at Site 4. The highest biomass values were found at Site 1. The highest total yearly biomass was measured in 2018 and reached 6.3 × 102 gwwt m−2.
Codium fragile was recorded in the summers of 2011 and 2013 at Site 1, with a biomass peak of 128 gwwt m−2 in 2013. In the springs of 2016 and 2018, few thalli were observed with negligible biomass. Grateloupia turuturu was detected every year except in 2014. It was observed at Site 1, mainly in winter, but sometimes in spring and autumn, with few thalli attached to plastic nets. The highest total yearly biomass was measured in spring 2013 and reached 75.3 gwwt m−2.
Agardhiella subulata was recorded, with very few thalli, at Site 4 only in spring 2016 and in winter 2017, when the highest total yearly biomass was reached (i.e., 18.2 gwwt m−2). Very few tetrasporic thalli of A. taxiformis (i.e., Falkenbergia rufolanosa) were collected for the first time in the summer of 2016 at Site 1 and, then, in the autumn of 2021. It was then detected at Site 2 in the summers of 2018, 2020, and 2021, and in winter 2021. Caulerpa cylindracea was found, with undetectable biomass, only in summer 2013 at Site 4. Solieria filiformis was also observed only once, with undetectable biomass, at Site 4 in spring 2017.
3.2 Molecular analysis
Some of the analyzed specimens were attributed to non-indigenous species that are difficult to recognize based only on morphology. For two species, the molecular analyses confirmed their belonging to NIS already reported in the study area such as G. minima (four specimens sequenced), and P. morrowii (two specimens sequenced). A newly introduced NIS was detected (three specimens sequenced) (Table 2), that is Neopyropia koreana, reported for the first time in the Mar Piccolo of Taranto.
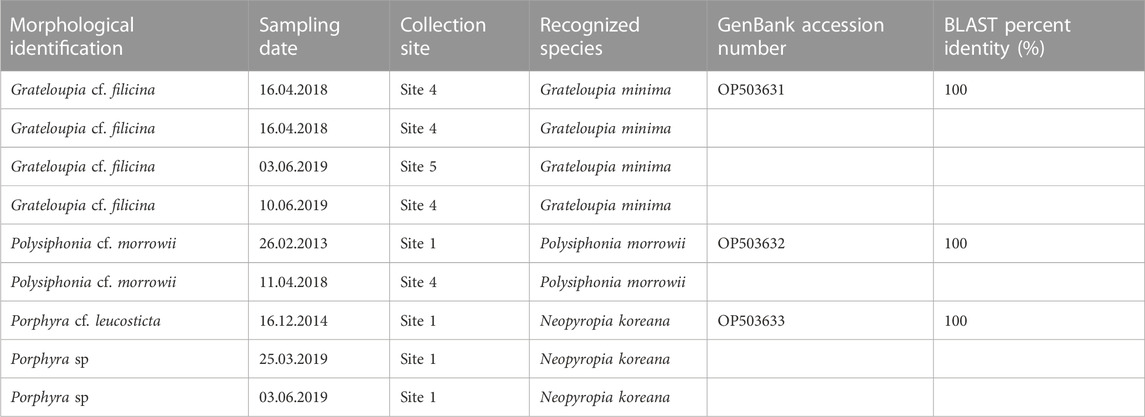
TABLE 2. List of NIS detected in the Mar Piccolo of Taranto with the DNA barcoding method. For each specimen, the recognized species and the highest BLAST percent identity are reported.
3.3 Multivariate analysis of the seaweed’s assemblages
PERMANOVA analysis showed a significant difference in the seaweed assemblages for the Site factor and both interactions between “Year × Site” and “Season × Site” (Tables 3, 4). Site 1 was separated from the others, as detected by nMDS ordination and PCoA (Figures 3, 4), although the variance explained by the first two axes was low (PCo1 = 25.1% and PCo2 = 14.7%). The main species positively correlated to the first axis (rs > 0.35) were Dictyota dichotoma var. intricata, Ellisolandia elongata, Amphiroa beauvoisii, and C. peregrina (NIS), which were associated with Site 1 (Supplementary Table S2). Replications of sites 3 and 4 showed a gradient of separation along the PCo2 axis, confirmed by the post hoc test (Table 4). Hypnea corona (NIS), Alsidium corallinum, and Spyridia filamentosa were the main species correlated to Site 3, while C. acicularis, Gelidium crinale, and C. okamurae (NIS) were characteristic of Site 4.
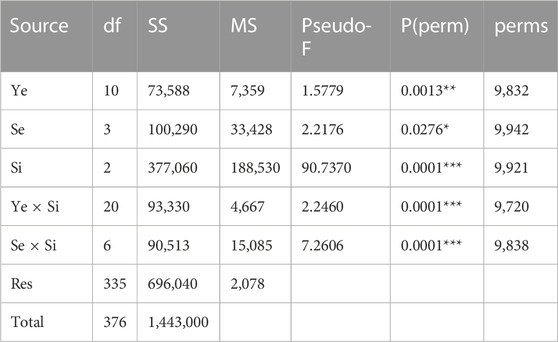
TABLE 3. Summary of PERMANOVA test based on Bray-Curtis similarity for four-root transformed biomass data on the basis of a multifactorial model with 3 factors (Year, Ye, Season, Se, Site, Si). The probabilities of each Pseudo-F value were obtained with 9,999 permutations of residuals under a reduced model. In red significant p-value, with significance levels coded by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001).
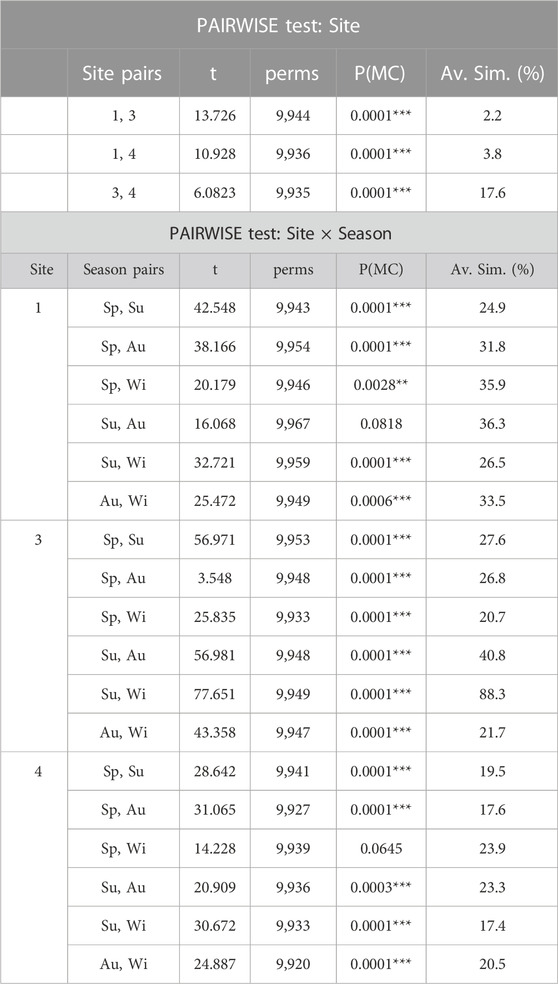
TABLE 4. Summary of PAIRWISE t-test among levels of Site and Season × Site interaction, with the seasons levels within the sites (Spring Sp, Summer Su, Autumn Au, Winter Wi). Values t-test (t), number of permutations (perms), Monte Carlo p-values (PMC) and Average Similarity (Av. Sim. %) between seasons pairs are reported. In red significant p-value, with significance levels coded by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001).
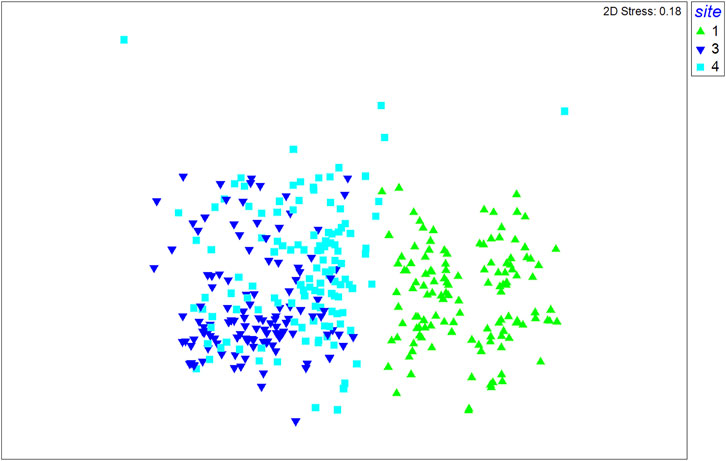
FIGURE 3. Nonmetric multidimensional scaling ordination (nMDS) based on the Bray–Curtis similarity matrix with the sites marked in the plot (1 = green; 3 = dark blue; 4 = light blue).
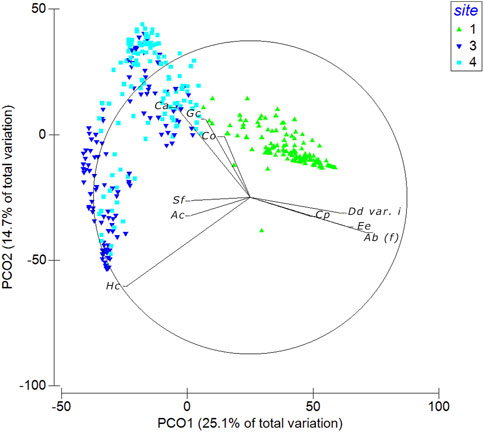
FIGURE 4. Principal Coordinate Analysis ordination (PCoA) based on the Bray–Curtis similarity measure of the stations labeled as sites (1 = green; 3 = dark blue; 4 = light blue). The percentages of explained variance (%) of both PCO1 and PCO2 axes are reported, and vectors indicate the main correlated species with the two axes (rs > 0.35). Species’ names are coded as: Ac, Alsidium corallinum; Ab (f), Amphiroa beauvoisii (f); Co, Caulacanthus okamurae; Cl, Chaetomorpha linum; Ca, Chondracanthus acicularis; Cp, Colpomenia peregrina; Co (f), Corallina officinalis (f); Dd var. d, Dictyota dichotoma var. dichotoma; Dd var. i, Dictyota dichotoma var. intricata; Ee, Ellisolandia elongata; Gc, Gelidium crinale; Gg (t), Gracilaria gracilis (t); Gt, Grateloupia turuturu; Hc, Hypnea corona; Pp, Padina pavonica; Pm, Polysiphonia morrowii; Sf, Spyridia filamentosa.
Considering the “Season × Site” interaction, differences in the seasonal pattern within the sites were detected by post hoc test and the centroids’ ordinations (Table 4; Figure 5; Supplementary Figure S1). In the PCoA, the sites were distributed along the first axis (55.2% of explained variance), with Site 1 characterized by different seasonal assemblages composed by D. dichotoma var. intricata, D. dichotoma var. dichotoma, E. elongata, G. turuturu (NIS), Corallina officinalis, Padina pavonica, A. beauvoisii, P. morrowii (NIS), C. peregrina (NIS) (correlation to PCo1, rs > 0.75; Supplementary Table S2). Along the second axis (21.6% of explained variance), Site 3 assemblage showed the greatest differences between seasons, with H. corona (NIS) as the typical species in summer and autumn, while A. corallinum and Chaetomorpha linum were abundant in spring. In winter, Site 3 assemblage was characterized by C. acicularis. In Site 4, small but significant changes among the seasons were observed, except for the winter-spring season pair, with Gracilaria gracilis, C. okamurae (NIS), and G. crinale as characteristic of these seasons.
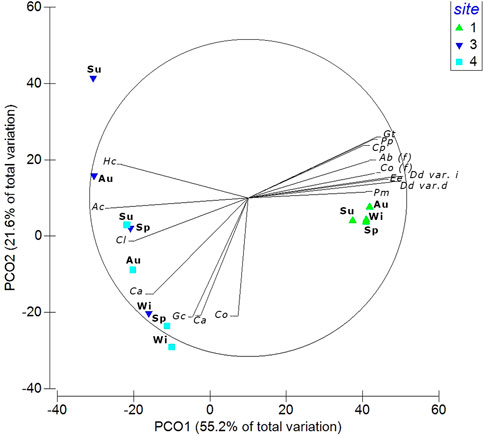
FIGURE 5. Principal Coordinate Analysis ordination (PCoA) based on the Bray–Curtis similarity measure of centroids calculated for the Season × Site interaction. The percentages of explained variance (%) of both PCo1 and PCo2 axes are reported, and vectors indicate the main correlated species with the two axes (rs > 0.75). Seasons are coded in the labels (Sp, Spring; Su, Summer; Au, Autumn; Wi, Winter). Species’ names are coded as: Ac, Alsidium corallinum; Ab (f), Amphiroa beauvoisii (f); Co, Caulacanthus okamurae; Cl, Chaetomorpha linum; Ca, Chondracanthus acicularis; Cp, Colpomenia peregrina; Co (f), Corallina officinalis (f); Dd var. d, Dictyota dichotoma var. dichotoma; Dd var. i, Dictyota dichotoma var. intricata; Ee, Ellisolandia elongata; Gc, Gelidium crinale; Gg (t), Gracilaria gracilis (t); Gt, Grateloupia turuturu; Hc, Hypnea corona; Pp, Padina pavonica; Pm, Polysiphonia morrowii; Sf, Spyridia filamentosa.
Considering the “Year × Site” interaction, the distance among centroids confirmed the separation of sites in the nMDS plot, resulting in line with post hoc test results (Supplementary Figure S2; Supplementary Table S3). Indeed, the seaweed assemblages of Site 1 were more similar in terms of year (centroids close among them), while a higher temporal variability in the assemblages was observed in Sites 3 and 4 (increase in the distance among centroids). Site 3 showed the highest variability in the assemblages over time, and H. corona was the most important NIS to contribute to seasonal changes. The remaining post hoc tests on seasons and year pairs are reported in Supplementary Table S3.
4 Discussion
Over the past 11 years, long-term observations have allowed for the detection of an increasing number of seaweed NIS in the Mar Piccolo of Taranto, confirming the susceptibility of this basin to biopollution. The Mar Piccolo is a very important place for mussel farming and shellfish imports, mainly consisting of Japanese oysters from Atlantic waters, which are supposed to be the main introduction vector for most seaweed NIS (Manghisi et al., 2010; Verlaque and Breton, 2019). In the Mar Piccolo, local mussel farmers’ observations have confirmed the introduction of U. pinnatifida since the 1990s through imported oysters (Cecere et al., 2000).
With a total number of 16 non-indigenous seaweeds recorded in the last 35 years, the Mar Piccolo basin is confirmed as the third seaweed NIS hot spot among the Mediterranean transitional water systems, after the Thau Lagoon and the Venice Lagoon (Boudouresque et al., 2020). These seaweed species show different behaviors mainly linked to their area of origin and their eco-physiological requirements. Therefore, different fates can be foreseen for each of them in the next years.
The warm tolerant H. corona (Rhodophyta, Gigartinales), which is the most abundant NIS in the basin until now, has proved to be the only NIS that behaves as an invasive species since it was first detected in 2000 (identified as H. cornuta). It spread into two zones of the Mar Piccolo Second Inlet (Cecere et al., 2016), seasonally monitored within the framework of LTER activities, until 2011. In 2016, observations along the NE coast of the basin showed the presence of H. corona in considerable amounts also in another zone, and in response, a new sampling site was added to the routine monitoring. According to recent studies, this species is part of the H. cornuta complex and has a tropical and subtropical distribution (Jesus de et al., 2019; Huisman et al., 2021). This indicates its long-lasting establishment in the increasingly warm Mar Piccolo seawaters, and, at the same time, enables us to make previsions on its permanence and further spread in the basin. The present study has confirmed that H. corona is widespread in the Mar Piccolo, likely enhanced by the high production of stellate propagules, which are an important means of vegetative propagation (Cecere et al., 2004).
Eight NIS species, native from cold-temperate oceanic waters, either in the Pacific or Atlantic Ocean, can be considered established in Mar Piccolo, ups and downs of biomass. Caulacanthus okamurae, C. peregrina, Neopyropia koreana, and P. morrowii have optimum growth temperatures between 18°C and 23°C (Kudo and Masuda, 1981; Vandermeulen, 1986; Choi et al., 2001; Choi and Nam, 2001; Croce and Parodi, 2017; Kim et al., 2022). Starting from 2011, the temperature in the Mar Piccolo seawaters generally exceeded 27°C in the warmest week, and, in recent years, these values have persisted for several weeks (Petrocelli et al., 2020a). At the same time, ever more frequent heat waves have affected wide areas around the world (Mentaschi et al., 2017). Therefore, for these species limited growth and their eventual disappearance in the long run, are predictable in the Mar Piccolo basin. For C. multifida, G. minima, and Osmundea oederi there is no information about the environmental requirements for their growth, so it is hard to gain an understanding of the reasons for fluctuations in their biomass as well as to foresee the fate of their populations in the basin. Concerning C. multifida, since the species has been present in the Mediterranean for a long time (Cormaci et al., 2012), we can also hypothesize that it has adapted to this environment and, therefore, it will become a constant element of the Mar Piccolo macrobenthic flora.
Grateloupia turuturu showed a first phase of population growth in the Mar Piccolo of Taranto, followed by a considerable fall, up to the current vestigial community (Petrocelli et al., 2020a). This species is considered among the most dangerous invasive seaweeds, due to its ability to replace both animal and plant native species on hard substrata in coastal ecosystems (Freitas et al., 2016), taking particular advantage in the absence of well-structured benthic communities (Mulas and Bertocci, 2016). Recent studies using an Ecological Niche Model based on ecophysiological responses forecast its future geographical distribution in temperate and warm-temperate seawaters (Koerich et al., 2020). Indeed, G. turuturu tolerates wider ranges of both temperature and salinity and nutrient concentrations up to eutrophication (Simon et al., 2001), which explains its permanence in the Mar Piccolo 15 years after its first record. No detrimental effect on the local communities has been observed to date, despite the presence of predisposing conditions.
Five NIS species were sporadically observed. Few thalli of C. fragile were not continuously observed. We suggest that these were most probably separate introductions, without a propagule pressure that can sustain its establishment. Codium fragile is considered a warm-temperate species with optimal growth temperatures between 21°C and 24°C (Fralick and Mathieson, 1973), and temperature is the most important environmental factor controlling its seasonal growth (Hanisak, 1979). Therefore, we suppose that C. fragile will never establish in the Mar Piccolo, due to the currently reported temperatures higher regimes than the optimal growth ones. Agardhiella subulata was particularly abundant in the Mar Piccolo from the end of the 1980s up to the second half of the 1990s (Cecere et al., 1992). Afterward, it underwent a complete disappearance, until sporadic new findings were recorded during this study, most probably due to different secondary re-introductions through shellfish import (Petrocelli et al., 2013), as already hypothesized in the Ganzirri Lake (Sicily, Ionian Sea) (Manghisi et al., 2010). Both male and female gametophytic thalli were collected in 2011, but to date, A. subulata has not been re-established in the Mar Piccolo. This species, preferentially distributed in shallow and sheltered environments, is native to the cold temperate Atlantic Ocean. P and N in seawater are limiting growth factors (Chopin et al., 1990). Agardhiella subulata is well established in the Venice lagoon, where temperatures can rise to 32.5°C (Sfriso et al., 2020), and was observed as a dominant species in the Po Delta lagoons, where high P and N concentrations were recorded (Sfriso et al., 2016). Therefore, temperature could seem less important than nutrients in fostering A. subulata settlement and establishment in the Mar Piccolo. Indeed, in the last 20 years a trend of temperature increase has been recorded (Cecere et al., 2016), but a considerable improvement in seawater quality was also observed in the basin, due to the closure of several urban sewage disposals (Kralj et al., 2016); this can be a disadvantage for A. subulata establishment in the basin.
In the studied period, negligible quantities of the tetrasporophyte (stage Falkenbergia) of Asparagopsis sp. were occasionally collected in the First Inlet. The genus Asparagopsis has still unsolved taxonomic questions and, thus, for the correct identification of species, the DNA barcoding method is imperative (Andreakis et al., 2007). Unfortunately, up to now, not enough quantities of Asparagopsis have been collected in the Mar Piccolo to perform molecular analysis. Therefore, in this study, we refer to Asparagopsis cf. taxiformis, since this species was reported for the first time in the basin in 2014 according to morphological identification (Bottalico et al., 2015). However, it is worth remembering that the congeneric NIS Asparagopsis armata Harvey is present in the nearby Mar Grande (Cecere et al., 1996). Solieria filiformis was the first NIS recorded in the Mar Piccolo. Indeed, its presence in the basin was already witnessed in a herbarium sheet dating back to 1922 (identified as Gracilaria confervoides Greville) (Perrone and Cecere, 1994). However, the first record of this species, which was also the first report for the Mediterranean, was based on a collection in 1987 (Cecere, 1990) when several unattached floating and sterile thalli of Solieriaceae (including A. subulata and S. filiformis) were sampled in both the basin inlets. Since then, recurring observations have shown that S. filiformis has spread considerably in the Mar Piccolo, becoming one of the dominant species in summer in the Second Inlet (Cecere et al., 1992). At the end of the 1990s, S. filiformis disappeared from the basin, because it was used in an experimental cultivation with mussels, starting in 1994, which led to the massive harvest of thalli (Cecere and Petrocelli, 2009). Solieria filiformis growth is strongly enhanced by a high content of N, mainly ammonium (Peñuela et al., 2018). During this study, in spring 2017, few sterile thalli were collected in the Mar Piccolo, most likely due to a new introduction event, not followed by the species re-establishment. The failure to find again this species in the following years could be due to a reduced propagule pressure, combined with the lower N content in seawater (Kralj et al., 2016). Caulerpa cylindracea was detected for the first time in the Mar Piccolo in 2001 (reported as Caulerpa racemosa), and continuously until 2004 by research projects focused solely on NIS (E. Cecere and A. Petrocelli unpublished data). It was widely distributed in the First Inlet, with some spots in the north-eastern part of the Second Inlet, at a depth between 0.5 and 3 m. Since then, a few filaments of C. cylindracea were collected again as part of this study in summer 2013. In the Tyrrhenian Sea, in situ studies showed that C. cylindracea is a nitrophilic species, but while N does not represent a limitative nutrient, P is limitative (Gennaro et al., 2015). Light is another limiting environmental factor for C. cylindracea growth. This species has elevated photosynthetic plasticity, but the energetic costs required for the acclimation often reduce its survival at low light conditions (Bernardeau-Esteller et al., 2015). These could have been the main factors leading to the disappearance of C. cylindracea in the Mar Piccolo, where urban effluents were mainly closed, but sedimentation remained very high (Bellucci et al., 2016). Caulerpa cylindracea is considered the first among the ten most invasive non-indigenous seaweed species in the Mediterranean Sea, with both negative and positive impacts on the invaded communities (Tsirintanis et al., 2022). However, for the moment, it can be considered another unsuccessful NIS in the Mar Piccolo of Taranto.
The DNA barcoding method has proved a very useful investigative tool for species identification, as it has enabled the identification of several other doubtful taxa present in the basin. Indeed, within the framework of the National Biodiversity Future Center (NBFC), funded by the Italian National Recovery and Resilience Plan (PNRR), a specific task is devoted to this issue in the future. Concerning G. minima, the molecular analyses helped the inclusion of morphologically different specimens under the same taxon. For P. morrowii, the first report for the Mar Piccolo was tentatively attributed to this taxon, because all the specimens collected in the Mediterranean Sea belonged to this species (Petrocelli et al., 2013). However, a misidentification with Polysiphonia senticulosa Harvey (Kudo and Masuda, 1981) could not be excluded. Polysiphonia morrowii was very probably present as an epiphyte on several other seaweeds: for some seaweed specimens the sequencing signal was disturbed, indicating the presence of more than one species (despite our efforts to clean the samples before the molecular analyses), and thus these results could not be included in this paper. However, it is worth underlining that, based on the BLAST search, the readable part of the sequence of each of these specimens had a 100% identity with P. morrowii sequences. Finally, it was possible to solve the misidentification of Neopyropia koreana with other congeneric species, commonly based only on morphological features until now (Kim et al., 2022).
The multivariate analysis results highlight the absence of long-term changes in the structure of seaweed assemblages, indicating no relevant disturbances due to NIS settlement in the Mar Piccolo. Indeed, most NIS species seem to follow a seasonal cycle in equilibrium with native species. This condition is particularly evident in Site 1, where the assemblage seems to maintain stability during the entire year, driven by high abundances of Corallinales, Dictyotales, and the regular occurrence of some NIS, as C. peregrina and P. morrowii. The main differences detected in the analyses could be affected by the spatial positions of investigated sites, with the assemblage in the First Inlet of Mar Piccolo (Site 1) being different from other sites in the Second Inlet (Sites 3 and 4). In the last two sites, the difference between the assemblages arises at a seasonal scale, with Site 3 showing the most relevant seasonal differences due to the high abundance of H. corona in the summer. This observation is in line with the studies on the seasonal cycle of this species in the Mar Piccolo (Cecere et al., 2016; Petrocelli et al., 2019). On the other hand, Site 4 showed a pattern of small changes in the macroalgal assemblages, with the summer-autumn and winter-spring periods showing slightly different results, and the latter being characterized by C. okamurae as the most important NIS, as observed in previous analyses (Petrocelli et al., 2020b).
Since 2011, a substantial increase in imported bivalve mollusks has been registered at the Taranto market. This was mainly due to a strong decline in local production, caused by the detection of organic pollutants beyond the limits permitted by current legislation (Cecere et al., 2016). Once they arrive by track, adult organisms (for direct sale) and juveniles (for fattening) were both indiscriminately immersed in the Mar Piccolo, also in contravention of European law (Cecere et al., 2016). During an informal conversation, two local shellfish importers and sellers reported that 200,000 quintals of bivalve mollusks officially reached the Taranto market in 2021. Moreover, they outlined that this value is surely underestimated due to the massive amount of product that is unlawfully imported, which could be estimated at around 500,000 quintals. Most of the imported bivalve mollusks (mainly mussels and clams) came from the Northern Adriatic, Greece, and Spain. Only a small percentage (mainly oysters) was imported from France. Most likely, the imported organisms have contributed to the continuous NIS introduction in the Mar Piccolo assessed in this study, as has already been demonstrated in other world zones (Mineur et al., 2007; 2014; Wolf et al., 2018). In this respect, continuous dialogue among researchers, mollusk producers, and local authorities has recently led the mayor of Taranto to enact a specific issue that bans juveniles and adults of allochthonous bivalve mollusks from the Mar Piccolo, to preserve both the original animal genetic pool from hybridization and the environment from the introduction of new NIS.
Higher quantities of imported products can also explain the significant differences among sites, with the separation of Site 1 in the First Inlet. Indeed, there are numerous bivalve mollusk retailers, restaurants, and immersed packets near this site, containing adult bivalve mollusks (mainly oysters), which have often been observed, with heaps of shells discarded there. The situation is different in the other investigated sites located in the Second Inlet, which are instead characterized by the presence of farming plants where the imported juvenile products are improvidently immersed for fattening. This could explain similarities.
Based on the current study, the Mar Piccolo seems not particularly suitable for NIS settlement and development. Most NIS native to cold-temperate zones have either disappeared or did not succeed in forming luxurious populations. The only exception is H. corona, a warm-tolerant species well adapted to the increasing seawater temperatures of the basin. Further systematic seasonal LTER observations will allow for the continuous monitoring of the situation and the early detection of any new NIS. Recently, the relevance of these activities was sealed by the Italian PNRR, which funded the enhancement of the e-LTER infrastructure, to which the Mar Piccolo belongs, thereby ensuring the continuation of data collection over time.
Data availability statement
The sequences produced in this study can be found in the INSDC online repositories. To access the sequences use the accession numbers OP503631, OP503632, and OP503633 in the search box that can be found at: https://www.ncbi.nlm.nih.gov/genbank/.
Author contributions
AP: conceptualization, investigation, data curation, writing—original draft, writing—review and editing. MW: molecular analyses, writing—original draft, and writing–review. KS: molecular analyses, writing—original draft, and writing–review. AS: writing–review. FR: statistical analysis and writing–review. PR: statistical analysis and writing—review. EC: investigation, data curation, and writing–review. All authors contributed to the article and approved the submitted version.
Funding
The authors acknowledge the support of activities from the Apulian Region, through POR PUGLIA FESR-FSE 2014/2020—Axis VI, Action 6.5 “Interventions for protection and valorization of marine and terrestrial biodiversity” Sub Action 6.5.a., and funded by EU-NextGenerationEU within the National Biodiversity Future Center, Project CN00000033.
Acknowledgments
This research was carried out within the framework of the LTER network (www.lteritalia.it). Giuseppe Portacci continuously and actively contributed to the sampling and sorting activities. The suggestions of the two referees are warmly acknowledged.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2023.1075458/full#supplementary-material
References
Akiyama, K., and Kurogi, M. (1982). Cultivation of Undaria pinnatifida (Harvey) Suringar, the decrease in crops from natural plants following crop increase from cultivation. Bull. Tohoku Reg. Fish. Res. Lab. 44, 91–100.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. doi:10.1016/S0022-2836(05)80360-2
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46. doi:10.1046/j.1442-9993.2001.01070.x
Anderson, M. J., Gorley, R. N., and Clarke, K. R. (2008). PERMANOVA+ for PRIMER: Guide to software and statistical methods. Plymouth: PRIMER-E Ltd.
Andreakis, N., Procaccini, G., Maggs, C., and Kooistra, W. H. (2007). Phylogeography of the invasive seaweed Asparagopsis (Bonnemaisoniales, Rhodophyta) reveals cryptic diversity. Mol. Ecol. 16, 2285–2299. doi:10.1111/j.1365-294X.2007.03306.x
Bellucci, L. G., Cassin, D., Giuliani, S., Botter, M., and Zonta, R. (2016). Sediment pollution and dynamic in the Mar Piccolo of Taranto (southern Italy): Insights from bottom sediment traps and surficial sediments. Environ. Sci. Pollut. Res. 23, 12554–12565. doi:10.1007/s11356-016-6738-6
Bernardeau-Esteller, J., Ruiz, J. M., Tomas, F., Sandoval-Gil, J. M., and Marín-Guirao, L. (2015). Photoacclimation of Caulerpa cylindracea: Light as a limiting factor in the invasion of native Mediterranean seagrass meadows. J. Exp. Mar. Biol. Ecol. 465, 130–141. doi:10.1016/j.jembe.2014.11.012
Bottalico, A., Russo, C., and Pati, R. (2015). Sulla presenza del genere Asparagopsis Montagne (Bonnemaisoniales, Rhodophyta) in Puglia (Italia meridionale). Inf. Bot. Ital. 47, 291–313.
Boudouresque, Ch.-F., Blanfuné, A., Pergent, G., Pergent-Martini, C., Perret-Boudouresque, M., and Thibaut, T. (2020). Impacts of marine and lagoon aquaculture on macrophytes in Mediterranean benthic ecosystems. Front. Mar. Sci. 7, 218. doi:10.3389/fmars.2020.00218
Cecere, E., Alabiso, G., Carlucci, R., Petrocelli, A., and Verlaque, M. (2016). Fate of two invasive or potentially invasive alien seaweeds in a central Mediterranean transitional water system: Failure and success. Bot. Mar. 59, 451–462. doi:10.1515/bot-2016-0053
Cecere, E., Cormaci, M., Furnari, G., Petrocelli, A., Saracino, O., and Serio, D. (1996). Benthic algal flora of Cheradi Islands, (Gulf of Taranto, Mediterranean Sea). Nova Hedwig. 62, 191–214.
Cecere, E., Moro, I., Wolf, M. A., Petrocelli, A., Verlaque, M., and Sfriso, A. (2011c). The introduced seaweed Grateloupia turuturu (Rhodophyta, Halymeniales) in two Mediterranean transitional water systems. Bot. Mar. 54, 23–33. doi:10.1515/bot.2011.009
Cecere, E., Petrocelli, A., Portacci, G., Mineur, F., and Verlaque, M. (2011b). Grateloupia minima (Rhodophyta, Gigartinales) in the Thau lagoon and in the Mar Piccolo of Taranto: First report for the Mediterranean Sea. Boll. Musei Ist. Biol. Univ. Genova 73, 78.
Cecere, E., Petrocelli, A., and Saracino, O. D. (2000). Undaria pinnatifida (Fucophyceae, Laminariales) spread in the central Mediterranean: Its occurrence in the Mar Piccolo of Taranto (Ionian Sea, southern Italy). Cryptogam. Algol. 21, 305–309. doi:10.1016/S0181-1568(00)00113-6
Cecere, E., and Petrocelli, A. (2009). “The Mar Piccolo of Taranto,” in Flora and vegetation of the Italian transitional water systems. Editors E. Cecere, A. Petrocelli, G. Izzo, and A. Sfriso (Spinea, Venice: CoRiLa, Stampa Multigraf), 195–227.
Cecere, E., Petrocelli, A., and Verlaque, M. (2004). Morphology and vegetative reproduction of the introduced species Hypnea cornuta (Rhodophyta, Gigartinales) in the Mar Piccolo of Taranto (Italy), Mediterranean Sea. Bot. Mar. 47, 381–388. doi:10.1515/BOT.2004.056
Cecere, E., Portacci, G., Petrocelli, A., Wolf, M. A., Sciuto, K., and Moro, I. (2011a). “Corsi e ricorsi storici: Il ritorno dell’alga alloctona Agardhiella subulata (Rhodophyta, Gigartinales) nel Mar Piccolo di Taranto,” in Proceedings of Gruppo di Algologia riunione scientifica annuale (Ancona, Italy: CSI), 8.
Cecere, E., Saracino, O. D., Fanelli, M., and Petrocelli, A. (1992). Presence of a drifting algal bed in the Mar Piccolo basin, Taranto (Ionian Sea, southern Italy). J. Appl. Phycol. 4, 323–327. doi:10.1007/BF02185789
Cecere, E. (1990). Sulla presenza nel Golfo di Taranto di una specie nuova per il Mediterraneo: Solieria filiformis (Kützing) Gabrielson (Rhodophyta, Gigartinales). Oebalia 16, 629–631.
Choi, H. G., and Nam, K. W. (2001). Growth, tetrasporogenesis, and life history in culture of Caulacanthus okamurae (Gigartinales, Rhodophyta) from Korea. Bot. Mar. 44, 315–320. doi:10.1515/BOT.2001.040
Choi, H. G., Nam, K. W., and Norton, T. A. (2001). No whirlwind romance: Typhoons, temperature and the failure of reproduction in Caulacanthus okamurae (Gigartinales, Rhodophyta). Eur. J. Phycol. 36, 353–358. doi:10.1080/09670260110001735498
Chopin, T., Hanisak, M. D., Koehn, F. E., Mollion, J., and Moreau, S. (1990). Studies on carrageenans and effects of seawater phosphorus concentration on carrageenan content and growth of Agardhiella subulata (C. Agardh) Kraft and Wynne (Rhodophyceae, Solieriaceae). J. Appl. Phycol. 2, 3–16. doi:10.1007/BF02179764
Clarke, K. R. (1993). Nonparametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18, 117–143. doi:10.1111/j.1442-9993.1993.tb00438.x
Clarke, K. R., and Warwick, R. M. (2001). Change in marine communities: An approach to statistical analysis and interpretation. Plymouth: PRIMER-E.
Compagnoni, A., Bibian, A. J., Ochocki, B. M., Levin, S., Zhu, K., and Miller, T. E. (2020). popler: An R package for extraction and synthesis of population time series from the long-term ecological research (LTER) network. Methods Ecol. Evol. 11, 258–264. doi:10.1111/2041-210X.13319
Copp, G. H., Templeton, M., and Gozlan, R. E. (2007). Propagule pressure and the invasion risks of non-native freshwater fishes: A case study in England. J. Fish. Biol. 71, 148–159. doi:10.1111/j.1095-8649.2007.01680.x
Cormaci, M., Furnari, G., Catra, M., Alongi, G., and Giaccone, G. (2012). Flora marina bentonica del Mediterraneo: Phaeophyceae. Boll. Accad. Gioenia Sci. Nat. Catania 45, 1–508.
Croce, M. E., and Parodi, E. R. (2017). The establishment of the non-native seaweed Polysiphonia morrowii in Northern Patagonia: Size of thallus and reproduction. Aquat. Bot. 136, 35–38. doi:10.1016/j.aquabot.2016.09.002
De Clerck, O., Gavio, B., Fredericq, S., Bárbara, I., and Coppejans, E. (2005). Systematics of Grateloupia filicina (Halymeniaceae, Rhodophyta), based on rbcL sequence analyses and morphological evidence, including the reinstatement of G. minima and the description of G. capensis sp. nov. J. Phycol. 41, 391–410. doi:10.1111/j.1529-8817.2005.04189.x
Fralick, R. A., and Mathieson, A. C. (1973). Ecological studies of Codium fragile in New England, USA. Mar. Biol. 19, 127–132. doi:10.1007/BF00353583
Freitas, C., Araújo, R., and Bertocci, I. (2016). Patterns of benthic assemblages invaded and non-invaded by Grateloupia turuturu across rocky intertidal habitats. J. Sea Res. 115, 26–32. doi:10.1016/j.seares.2016.07.002
Freshwater, D. W., and Rueness, J. (1994). Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia 33, 187–194. doi:10.2216/i0031-8884-33-3-187.1
Gennaro, P., Piazzi, L., Persia, E., and Porrello, S. (2015). Nutrient exploitation and competition strategies of the invasive seaweed Caulerpa cylindracea. Eur. J. Phycol. 50, 384–394. doi:10.1080/09670262.2015.1055591
Gower, J. C. (1966). Some distance properties of latent root and vector methods used in multivariate analysis. Biometrik 53, 325–338. doi:10.2307/2333639
Guerra-Castro, E. J., Conde, J. E., and Cruz-Motta, J. J. (2016). Scales of spatial variation in tropical benthic assemblages and their ecological relevance: Epibionts on caribbean mangrove roots as a model system. Mar. Ecol. Prog. Ser. 548, 97–110. doi:10.3354/meps11693
Hanisak, M. D. (1979). Growth patterns of Codium fragile ssp. tomentosoides in response to temperature, irradiance, salinity, and nitrogen source. Mar. Biol. 50, 319–332. doi:10.1007/BF00387009
Huisman, J. M., D’Archino, R., Nelson, W., Boo, S. M., and Petrocelli, A. (2021). Cryptic cryptogam revealed: Hypnea corona (Gigartinales: Cystocloniaceae), a new red algal species described from the Hypnea cornuta complex. Pac. Sci. 75, 263–268. doi:10.2984/75.2.8
Jesus de, P. B., Costa, A. L., Castro Nunes de, J. M., Manghisi, A., Genovese, G., Morabito, M., et al. (2019). Species delimitation methods reveal cryptic diversity in the Hypnea cornuta complex (Cystocloniaceae, Rhodophyta). Eur. J. Phycol. 54, 135–153. doi:10.1080/09670262.2018.1522454
Keser, M., Swenarton, J. T., and Foertch, J. F. (2005). Effects of thermal input and climate change on growth of Ascophyllum nodosum (Fucales, Phaeophyceae) in eastern Long Island Sound (USA). J. Sea Res. 54, 211–220. doi:10.1016/j.seares.2005.05.001
Kim, H.-S., Choi, H. G., Hwang, M. S., Jeon, Y. J., Yarish, C., and Kim, J. K. (2022). Concise review of the genus Neopyropia (Rhodophyta: Bangiales). J. Appl. Phycol. 34, 1805–1824. doi:10.1007/s10811-022-02776-1
Koerich, G., Assis, J., Burle Costa, G., Nasri Sissini, M., Serrão, E. A., Rubi Rörig, L., et al. (2020). How experimental physiology and ecological niche modelling can inform the management of marine bioinvasions? Sci. Tot. Environ. 700, 134692. doi:10.1016/j.scitotenv.2019.134692
Kralj, M., De Vittor, C., Comici, C., Relitti, F., Auriemma, R., Alabiso, G., et al. (2016). Recent evolution of the physical–chemical characteristics of a site of national interest—The Mar Piccolo of Taranto (Ionian Sea)—And changes over the last 20 years. Env. Sci. Pollut. Res. 23, 12675–12690. doi:10.1007/s11356-015-5198-8
Kudo, T., and Masuda, M. (1981). A taxonomic study of Polysiphonia morrowii Harvey (Rhodophyta, Ceramiales). Jap. J. Phycol. 29, 263–272.
Manghisi, A., Morabito, M., Bertuccio, C., Le Gall, L., Couloux, A., Cruaud, C., et al. (2010). Is routine DNA barcoding an efficient tool to reveal introductions of alien macroalgae? A case study of Agardhiella subulata (Solieriaceae, Rhodophyta) in Cape Peloro lagoon (Sicily, Italy). Cryptogam. Algol. 31, 423.
Mentaschi, L., Vousdoukas, M. I., Voukouvalas, E., Dosio, A., and Feyen, L. (2017). Global changes of extreme coastal wave energy fluxes triggered by intensified teleconnection patterns. Geophys. Res. Lett. 44, 2416–2426. doi:10.1002/2016GL072488
Mineur, F., Belsher, T., Johnson, M. P., Maggs, C. A., and Verlaque, M. (2007). Experimental assessment of oyster transfers as a vector for macroalgal introductions. Biol. Conserv. 137, 237–247. doi:10.1016/j.biocon.2007.02.001
Mineur, F., Le Roux, A., Maggs, A., and Verlaque, M. (2014). Positive feedback loop between introductions of non-native marine species and cultivation of oysters in Europe. Conserv. Biol. 28, 1667–1676. doi:10.1111/cobi.12363
Montes, M., Rico, J. M., García-Vázquez, E., and Borrell, Y. J. (2016). Morphological and molecular methods reveal the Asian alga Grateloupia imbricata (Halymeniaceae) occurs on Cantabrian Sea shores (Bay of Biscay). Phycologia 55, 365–370. doi:10.2216/15-112.1
Montes, M., Rico, J. M., García-Vazquez, E., and Pichs, Y. J. B. (2017). Molecular barcoding confirms the presence of exotic Asian seaweeds (Pachymeniopsis gargiuli and Grateloupia turuturu) in the Cantabrian Sea, Bay of Biscay. PeerJ 5, e3116. doi:10.7717/peerj.3116
Mulas, M., and Bertocci, I. (2016). Devil’s tongue weed (Grateloupia turuturu Yamada) in northern Portugal: Passenger or driver of change in native biodiversity? Mar. Environ. Res. 118, 1–9. doi:10.1016/j.marenvres.2016.04.007
Newton, A., Icely, J., Cristina, S., Brito, A., Cardoso, A. C., Colijn, F., et al. (2014). An overview of ecological status, vulnerability and future perspectives of European large shallow, semi-enclosed coastal systems, lagoons and transitional waters. Estuar. Coast. Shelf Sci. 140, 95–122. doi:10.1016/j.ecss.2013.05.023
Peñuela, A., Robledo, D., Bourgougnon, N., Bedoux, G., Hernández-Núñez, E., and Freile-Pelegrín, Y. (2018). Environmentally friendly valorization of Solieria filiformis (Gigartinales, Rhodophyta) from IMTA using a biorefinery concept. Mar. Drugs 16, 487. doi:10.3390/md16120487
Perrone, C., and Cecere, E. (1994). Two solieriacean algae new to the Mediterranean: Agardhiella subulata and Solieria filiformis (Rhodophyta, Gigartinales). J. Phycol. 30, 98–108. doi:10.1111/j.0022-3646.1994.00098.x
Petrocelli, A., Acquaviva, M. I., Alabiso, G., Belmonte, M., Biandolino, F., Cardellicchio, N., et al. (2021). “IT22-M Mar Piccolo di Taranto,” in La Rete Italiana per la Ricerca Ecologica di Lungo Termine. Lo studio della biodiversità e dei cambiamenti. Editors L. Capotondi, M. Ravaioli, A. Acosta, F. Chiarini, A. Lami, A. Stanisciet al. (Roma, Italy: CNR Edizioni), 675–700. doi:10.5281/zenodo.5570272
Petrocelli, A., Alabiso, G., Cecere, E., Ricci, P., and Carlucci, R. (2020a). Invasive or not? The case of Grateloupia turuturu (Rhodophyta, Halymeniales) in the northern Ionian Sea (Mediterranean Sea). Mar. Pollut. Bull. 161, 111748. doi:10.1016/j.marpolbul.2020.111748
Petrocelli, A., and Cecere, E. (2010). Biodiversity and mollusc transfer: Need of observance of the laws to avoid alien seaweeds introduction. Biol. Mar. Mediterr. 17, 175–176.
Petrocelli, A., Cecere, E., and Rubino, F. (2019). Successions of phytobenthos species in a Mediterranean transitional water system: The importance of long term observations. Nat. Conserv. 34, 217–246. doi:10.3897/natureconservation.34.30055
Petrocelli, A., Cecere, E., and Verlaque, M. (2013). Alien marine macrophytes in transitional water systems: New entries and reappearances in a Mediterranean coastal basin. BioInvasions Rec. 2, 177–184. doi:10.3391/bir.2013.2.3.01
Petrocelli, A., Wolf, M. A., Cecere, E., Sciuto, K., and Sfriso, A. (2020b). Settlement and spreading of the introduced seaweed Caulacanthus okamurae (Rhodophyta) in the Mediterranean Sea. Diversity 12, 129. doi:10.3390/d12040129
Piñeiro-Corbeira, C., Verbruggen, H., and Díaz-Tapia, P. (2020). Molecular survey of the red algal family Rhodomelaceae (Ceramiales, Rhodophyta) in Australia reveals new introduced species. J. Appl. Phycol. 32, 2535–2547. doi:10.1007/s10811-019-01932-4
Pugnetti, A., Acri, F., Bernardi Aubry, F., Camatti, E., Cecere, E., Facca, C., et al. (2013). The Italian Long-Term Ecological Research (LTER-Italy) network: Results, opportunities, and challenges for coastal transitional ecosystems. Transit. Waters Bull. 7, 43–63. doi:10.1285/i1825229Xv7n1p43
Salamon, M., Lévêque, L., Ballenghien, M., and Viard, F. (2020). Spill-back events followed by self-sustainment explain the fast colonization of a newly built marina by a notorious invasive seaweed. Biol. Invasions 22, 1411–1429. doi:10.1007/s10530-019-02193-5
Sanderson, J. C. (1990). A preliminary survey of the distribution of the introduced macroalga Undaria pinnatifida (Harv) Suringar on the East Coast of Tasmania. Bot. Mar. 33, 153–158. doi:10.1515/botm.1990.33.2.153
Saunders, G. W. (2009). Routine DNA barcoding of Canadian Gracilariales (Rhodophyta) reveals the invasive species Gracilaria vermiculophylla in British Columbia. Mol. Ecol. Resour. 9, 140–150. doi:10.1111/j.1755-0998.2009.02639.x
Sfriso, A., Buosi, A., Wolf, M. A., and Sfriso, A. A. (2020). Invasion of alien macroalgae in the Venice Lagoon, a pest or a resource? Aquat. Invasions 15, 245–270. doi:10.3391/ai.2020.15.2.03
Sfriso, A., Facca, C., Bon, D., and Buosi, A. (2016). Macrophytes and ecological status assessment in the Po delta transitional systems, Adriatic Sea (Italy). Application of Macrophyte Quality Index (MaQI). Acta Adriat. 57, 209–225.
Simon, C., Ar Gall, E., and Deslandes, E. (2001). Expansion of the red alga Grateloupia doryphora along the coasts of Brittany. Hydrobiologia 443, 23–29. doi:10.1023/A:1017587918604
Trowbridge, C. D., Little, C., and Stirling, P. (2016). Post-proliferation population of introduced seaweed: Decline of a parthenogenetic green seaweed in Irish marine reserve. Biol. Environ. Proc. R. Ir. Acad. 116, 87–102. doi:10.3318/bioe.2016.10
Tsirintanis, K., Azzurro, E., Crocetta, F., Dimiza, M., Froglia, C., Gerovasileiou, V., et al. (2022). Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 17, 308–352. doi:10.3391/ai.2022.17.3.01
Turner, M. G., Collins, S. L., Lugo, A. L., Magnuson, J. J., Rupp, T. S., and Swanson, F. J. (2003). Disturbance dynamics and ecological response: The contribution of Long-Term Ecological Research. BioScience 53, 46–56. doi:10.1641/0006-3568(2003)053[0046:DDAERT]2.0.CO;2
Vandermeulen, H. (1986). Growth of Colpomenia peregrina (Phaeophyceae) in culture: Effects of salinity, temperature and daylength. J. Phycol. 22, 138–144. doi:10.1111/j.1529-8817.1986.tb04156.x
Verlaque, M., Boudouresque, Ch.-F., and Mineur, F. (2007). Oyster transfers as a vector for marine species introductions: A realistic approach based on the macrophytes. CIESM Workshop Monogr. 32, 39–48.
Verlaque, M., and Breton, G. (2019). Biological invasion: Long term monitoring of the macroalgal flora of a major European harbor complex. Mar. Pollut. Bull. 143, 228–241. doi:10.1016/j.marpolbul.2019.04.038
Verlaque, M., Ruitton, S., Mineur, F., and Boudouresque, Ch.-F. (2015). CIESM atlas of exotic species in the mediterranean. 4: Macropytes. Monaco: CIESM Publishers, 362.
Watanabe, Y., Nishihara, G. N., Tokunaga, S., and Terada, R. (2014). The effects of irradiance and temperature responses and the phenology of a native alga, Undaria pinnatifida (Laminariales), at the southern limit of its natural distribution in Japan. J. Appl. Phycol. 26, 2405–2415. doi:10.1007/s10811-014-0264-z
Wilkes, R. J., McIvor, L. M., and Guiry, M. D. (2005). Using rbcL sequence data to reassess the taxonomic position of some Grateloupia and Dermocorynus species (Halymeniaceae, Rhodophyta) from the north-easten Atlantic. Eur. J. Phycol. 40, 53–60. doi:10.1080/09670260400024634
Wolf, M. A., Buosi, A., Juhmani, A. S. F., and Sfriso, A. (2018). Shellfish import and hull fouling as vectors for new red algal introductions in the Venice Lagoon. Estuar. Coast. Shelf Sci. 215, 30–38. doi:10.1016/j.ecss.2018.09.028
Wolf, M. A., Sciuto, K., Maggs, C. A., Barros-Barreto, M. B., Andreoli, C., and Moro, I. (2011). Ceramium Roth (Ceramiales, Rhodophyta) from Venice lagoon (Adriatic Sea, Italy): Comparative studies of mediterranean and atlantic taxa. Taxon 60, 1584–1595. doi:10.1002/tax.606004
Wolf, M. A., Sfriso, A., and Moro, I. (2014). Thermal pollution and settlement of new tropical alien species: The case of Grateloupia yinggehaiensis (Rhodophyta) in the Venice Lagoon. Estuar. Coast. Shelf Sci. 147, 11–16. doi:10.1016/j.ecss.2014.05.020
Yamagishi, Y., Masuda, M., Abe, T., Uwai, S., Kogame, K., Kawaguchi, S., et al. (2003). Taxonomic notes on marine algae from Malaysia. XI. Four species of Rhodophyceae. Bot. Mar. 46, 534–547. doi:10.1515/BOT.2003.056
Zilioli, M., Oggioni, A., Tagliolato, P., Pugnetti, A., and Carrara, P. (2019). Feeding Essential Biodiversity Variables (EBVs): Actual and potential contributions from LTER-Italy. Nat. Conserv. 34, 477–503. doi:10.3897/natureconservation.34.30735
Zingone, A., D'Alelio, D., Mazzocchi, M. G., Montresor, M., and Sarno, D.LTER-MC team (2019). Time series and beyond: Multifaceted plankton research at a marine Mediterranean LTER site. Nat. Conserv. 34, 273–310. doi:10.3897/natureconservation.34.30789
Keywords: long term ecological research, Mar Piccolo, Mediterranean Sea, non-indigenous species, seaweeds, transitional water systems
Citation: Petrocelli A, Wolf MA, Sciuto K, Sfriso A, Rubino F, Ricci P and Cecere E (2023) Long-term data prove useful to keep track of non-indigenous seaweed fate. Front. Environ. Sci. 11:1075458. doi: 10.3389/fenvs.2023.1075458
Received: 20 October 2022; Accepted: 21 June 2023;
Published: 06 July 2023.
Edited by:
Caterina Bergami, National Research Council (CNR), ItalyReviewed by:
Pilar Diaz-Tapia, Spanish Institute of Oceanography (IEO), SpainLuigi Piazzi, University of Sassari, Italy
Copyright © 2023 Petrocelli, Wolf, Sciuto, Sfriso, Rubino, Ricci and Cecere. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonella Petrocelli, antonella.petrocelli@irsa.cnr.it
 Antonella Petrocelli
Antonella Petrocelli Marion Adelheid Wolf
Marion Adelheid Wolf Katia Sciuto
Katia Sciuto Adriano Sfriso
Adriano Sfriso Fernando Rubino
Fernando Rubino Pasquale Ricci
Pasquale Ricci Ester Cecere
Ester Cecere