Sexual Health Dysfunction After Radiotherapy for Gynecological Cancer: Role of Physical Rehabilitation Including Pelvic Floor Muscle Training
- 1Radiation Oncology Unit, Clinical Department, National Center for Oncological Hadrontherapy (CNAO), Pavia, Italy
- 2Department of Clinical, Surgical, Diagnostic and Paediatric Sciences, University of Pavia, Pavia, Italy
- 3Department of Obstetrics and Gynecology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 4Lincoln International Business School, University of Lincoln, Lincoln, United Kingdom
- 5Center of Organization and Governance of the Public Administration, University of Pavia, Pavia, Italy
- 6Ipazia, International Observatory on Gender Research, Rome, Italy
- 7Department of Sports Medicine, Norwegian School of Sports Sciences, Oslo, Norway
- 8Department of Obstetrics and Gynecology, Akershus University Hospital, Lorenskog, Norway
Introduction: The present study aims to describe: 1. How the side effects of radiotherapy (RT) could impact sexual health in women; 2. The effectiveness of physical rehabilitation including pelvic floor muscle training (PFMT) in the management of sexual dysfunction after RT.
Materials and Methods: Search keys on PubMed, Web of Science, Scopus, PEDro, and Cochrane were used to identify studies on women treated with radical or adjuvant RT and/or brachytherapy for gynecological cancers with an emphasis on vulvo-vaginal toxicities and PFMT studies on sexual dysfunction for this group of women.
Results: Regarding the first key question, we analyzed 19 studies including a total of 2,739 women who reported vaginal dryness, stenosis, and pain as the most common side effects. Reports of dosimetric risk factors and dose-effect data for vaginal and vulvar post-RT toxicities are scant. Only five studies, including three randomized controlled trials (RCTs), were found to report the effect of PFMT alone or in combination with other treatments. The results showed some evidence for the effect of training modalities including PFMT, but to date, there is insufficient evidence from high-quality studies to draw any conclusion of a possible effect.
Conclusions: Gynecological toxicities after RT are common, and their management is challenging. The few data available for a rehabilitative approach on post-actinic vulvo-vaginal side effects are encouraging. Large and well-designed RCTs with the long-term follow-up that investigate the effect of PFMT on vulvo-vaginal tissues and pelvic floor muscle function are needed to provide further guidance for clinical management.
Introduction
During the past two decades, advances in oncology, radiotherapy (RT), and surgery have significantly improved the survival rate of women affected by gynecological cancers (GyC) (1–6). In recent years, there has been an emerging interest in late treatment sequelae. Vulvodynia (7), dyspareunia (8, 9), fecal and urinary incontinence (10), lower limb lymphedema (11), hot flashes (12), fatigue (13), insomnia (12), and emotional distress (14) are frequently reported side effects that could considerably affect the quality of life (QoL) of GyC survivors (4, 15). Moreover, pelvic RT can lead to premature menopause in young women or to a worsening of menopausal syndrome with increased metabolic, cardiovascular, and osteoporotic risks (16). Not only women who underwent RT but also patients with ovarian cancers (17) experienced a reduction of sexual function after diagnosis and oncological treatment. However, several of these side effects appear as underreported and undertreated (18, 19) even though more than 40% of cancer survivors expressed interest in receiving sexual healthcare (20). Understanding and treating physical symptoms and the consequent psychological issues stand as primary challenges for the healthcare staff dealing with GyC survivors. RT studies usually describe radiation-induced organ-related morbidity (particularly for bladder, rectum, and bowel toxicities) with the aim to improve the dose optimization approach. Despite the impact on QoL of long-survivors and sexually active patients, the vagina has so far only slightly been included in the panel of organs at risk (OARs) in the RT planning treatment for GyC. To date, there is no consensus about vaginal dose constraints (19). The psychological stress of sexually related toxicities negatively impacts the QoL of GyC survivors who often report feelings of shame, inadequacy, emotional distancing from the partner, and alteration in body image (21–23).
Several studies have reported that external beam RT (EBRT) and brachytherapy (BT) could significantly affect the pelvic floor muscles due to the development of fibrosis in the smooth and the striated layer of the muscle tissue (24, 25) and, thus, lead to urinary incontinence, anal incontinence, and sexual distress. The long-term side effects of RT cause an alteration of vaginal structure, such as vaginal stenosis, conglutination, dryness, and dyspareunia. The pathological process of vaginal tissue damage appears as a decrease in vaginal length, in the elasticity of the muscles layer, and vaginal lubrication (26, 27). Meta-analysis, systematic reviews, and clinical trials have found that physical activities are effective in decreasing the state of systemic inflammation, reducing cancer-related-fatigue, and improving current QoL (28–36), but there are still few reports about rehabilitation and pelvic floor muscle training (PFMT) to alleviate vaginal and vulvar symptoms due to RT toxicities (37–39). This systematic review aims to evaluate:
• How the side effects of RT could impact the sexual health in women.
• The effectiveness of physical rehabilitation, including PFMT in the management of sexual dysfunction after RT.
Materials and Methods
Data Sources and Searches
A comprehensive literature search on the research questions was conducted during April 2021. PubMed, Web of Science, PEDro, and Scopus (40) were searched for published studies.
For studies on sexual health morbidity after RT in GyC survivors, the database search was done with a combination of the following keywords: “pelvic radiotherapy,” “toxicity,” “vaginal toxicity,” “vaginal brachytherapy,” “hadrontherapy,” “radiotherapy,” “rehabilitation,” “gynecological cancer,” “sexual health,” “quality of life”, “sexual dysfunction,” “pelvic floor,” including pluralization and US English/UK English spelling variations and suffixes/prefixes.
For studies on physical therapy, the search was performed with a combination of the following words: “pelvic floor,” “pelvic floor muscle,” “physiotherapy,” “training,” “exercise,” “education,” “dilator,” “physical therapy,” “pelvic radiotherapy,” “gynecological cancer,” “brachytherapy,” “toxicity,” “cancer,” “tumor,” including pluralization and US English/UK English spelling variations and suffixes/prefixes.
Study Selection and Data Extraction
We defined inclusion criteria for the literature search using the Population, Intervention, Control, Outcome, and Study (PICOS) design approach (41).
Patient Populations of Interest
We included studies of women with GyC treated with RT and/or BT, with or without concomitant chemotherapy in adjuvant and radical settings. We omitted studies considering re-irradiation or palliative RT.
Intervention and Control
The intervention for the first question was BT or/and RT as definitive or adjuvant therapy. We organized studies for our analysis considering the RT delivered (BT, EBRT, carbon-ion RT -CIRT-). We considered BT both employed as monotherapy or in combination with EBRT. The intervention for the second question was PFMT and physical activities aimed at reducing gynecological RT toxicities in women who had undergone RT for GyC (e.g., PFMT, vaginal massage, or dilator training). For PFMT, we considered a program of “repeated voluntary PFM contractions taught and supervised by a healthcare professional” (for example, PFMT for strengthening or relaxation, for urge suppression, single contractions to instantly control/prevent leakage) (42, 43). Individual or group PFMT, or relaxation training, with or without biofeedback were included.
Outcomes of Interest
The primary outcome measure for the first research question was that vaginal and vulvar post-RT toxicities were scored according to the Common Terminology Criteria for Adverse Events (CTCAE) scale (44), Radiation Therapy Oncology Group (RTOG) (45), and Dische score (46). Vulvodynia, when available, was assessed according to the ISSVD (International Society for the Study of Vulvovaginal Disease), ISSWSH (Boards of Directors of the International Society for the Study of Women's Sexual Health), and IPPS (International Pelvic Pain Society) Consensus Conference classification (47). Table 1 summarizes the vulvo-vaginal toxicities according to the CTCAE scoring system (44). For the second research question, PFM function, pain, and QoL were reported.
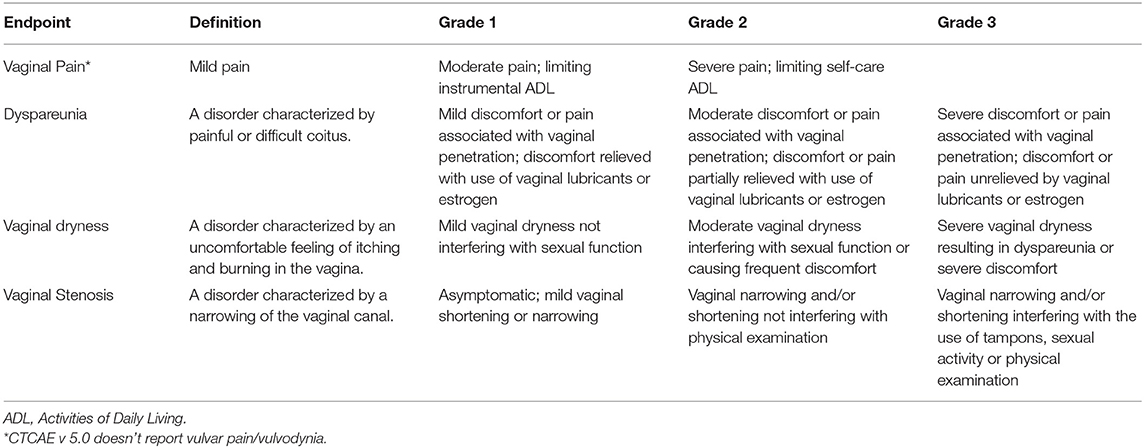
Table 1. Vulvo-vaginal morbidity after radiotherapy: definition and score according to Common Terminology Criteria for Adverse Events (CTCAE) v 5.0.
Study designs for the first research question were prospective cohort studies, cross-sectional studies, case-control, retrospective studies, and case series. Single case reports were excluded. For the second research question, both randomized controlled trials (RCTs) and uncontrolled trials were included. Case studies were excluded. In the case of duplicated datasets (e.g., multiple articles from the same study group or institution, related to the same treatment on the same cohort of the patient), only the manuscript with the most extended follow-up and the largest cohort was included.
Data Extraction and Quality Assessment
We screened the data, which included author names, publication year, study design characteristics, number of patients, age, histology, radiation technique and dose (total and for fraction), reported vaginal and vulvar toxicity, toxicity scale used, and follow-up time. For the second research question, we also extracted the intervention program type, duration, frequency, intensity, supervision, adherence, dropout, and outcomes.
Data Synthesis and Analysis
The flowcharts of the two literature analyses are displayed in Figures 1, 2. Studies are organized into two tables (Tables 2, 3). The RT toxicities analysis follows a descriptive analysis. We reported a descriptive analysis for the rehabilitation approach, and for RCT, the methodological quality of the studies was evaluated using the PEDro scale (Table 3). The PEDro method is a checklist of 10 items assessing the internal validity of clinical trials and 1 item assessing the external validity. The maximum possible score is 10/10 (excluding external validity item), with scores of ≥7 indicating high-quality study designs, while scores of 5–6 indicating moderate-quality study designs, and scores of <5 indicating low-quality study designs (70).
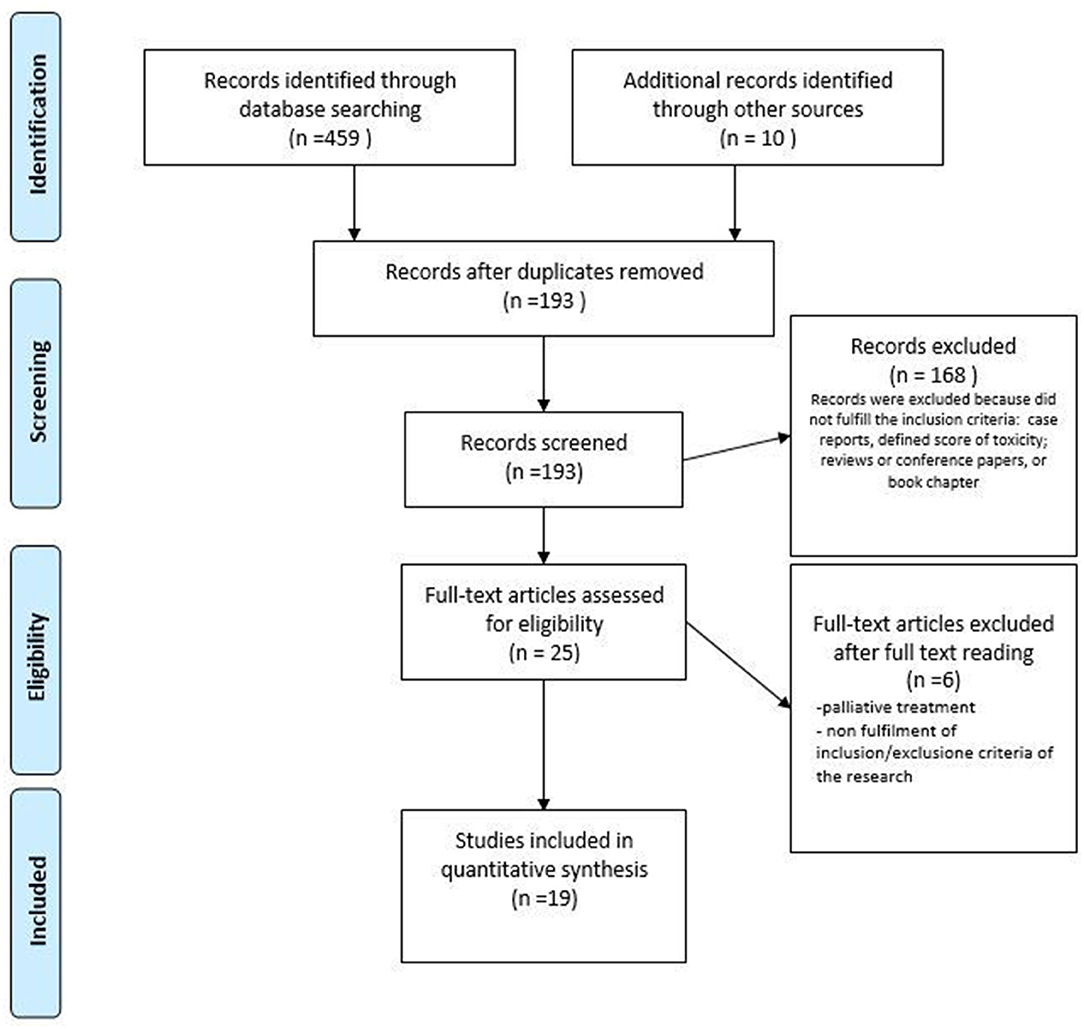
Figure 1. PRISMA flow diagram of the study selection process for vulvar and vaginal radiotherapy toxicity. Adapted from (48).
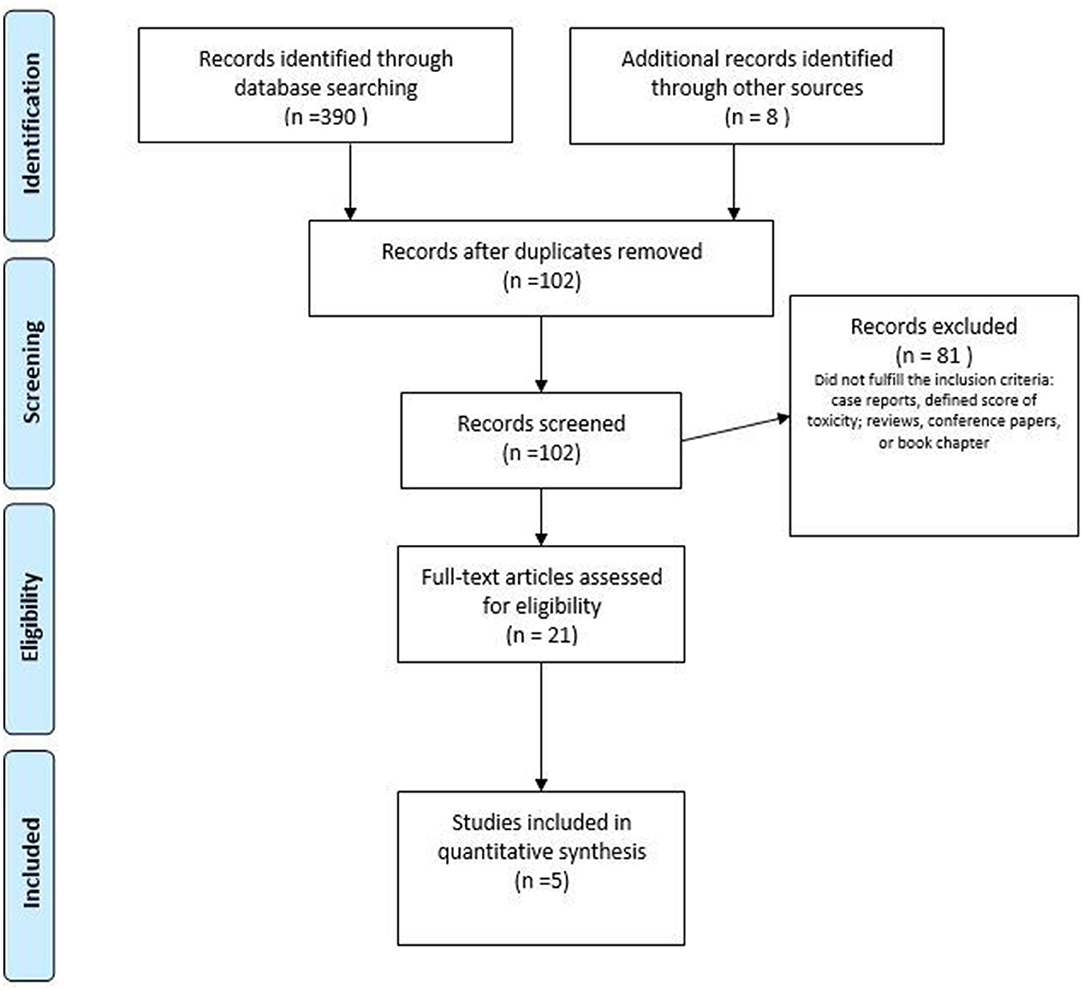
Figure 2. PRISMA flow diagram of the study selection process for pelvic floor muscle training (PFMT) as conservative management of sexual dysfunction after radiotherapy (RT). Adapted from (48).
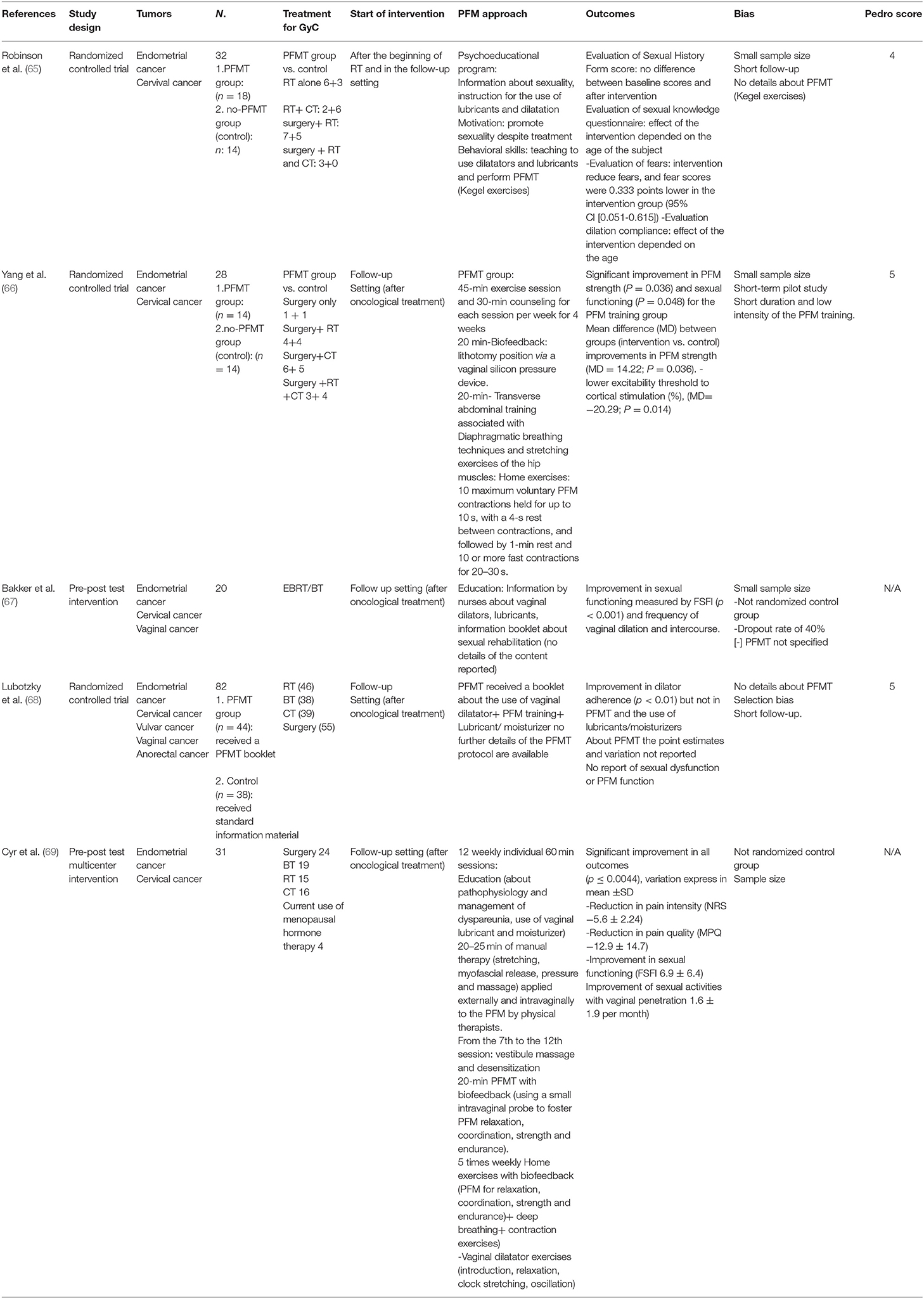
Table 3. Pelvic floor muscle training (PFMT) alone or in combination with other treatments for sexual dysfunction in gynecological cancer (GyC) patients.
Results
Key Question 1: How Could the Side Effects of RT Impact Sexual Health in Women?
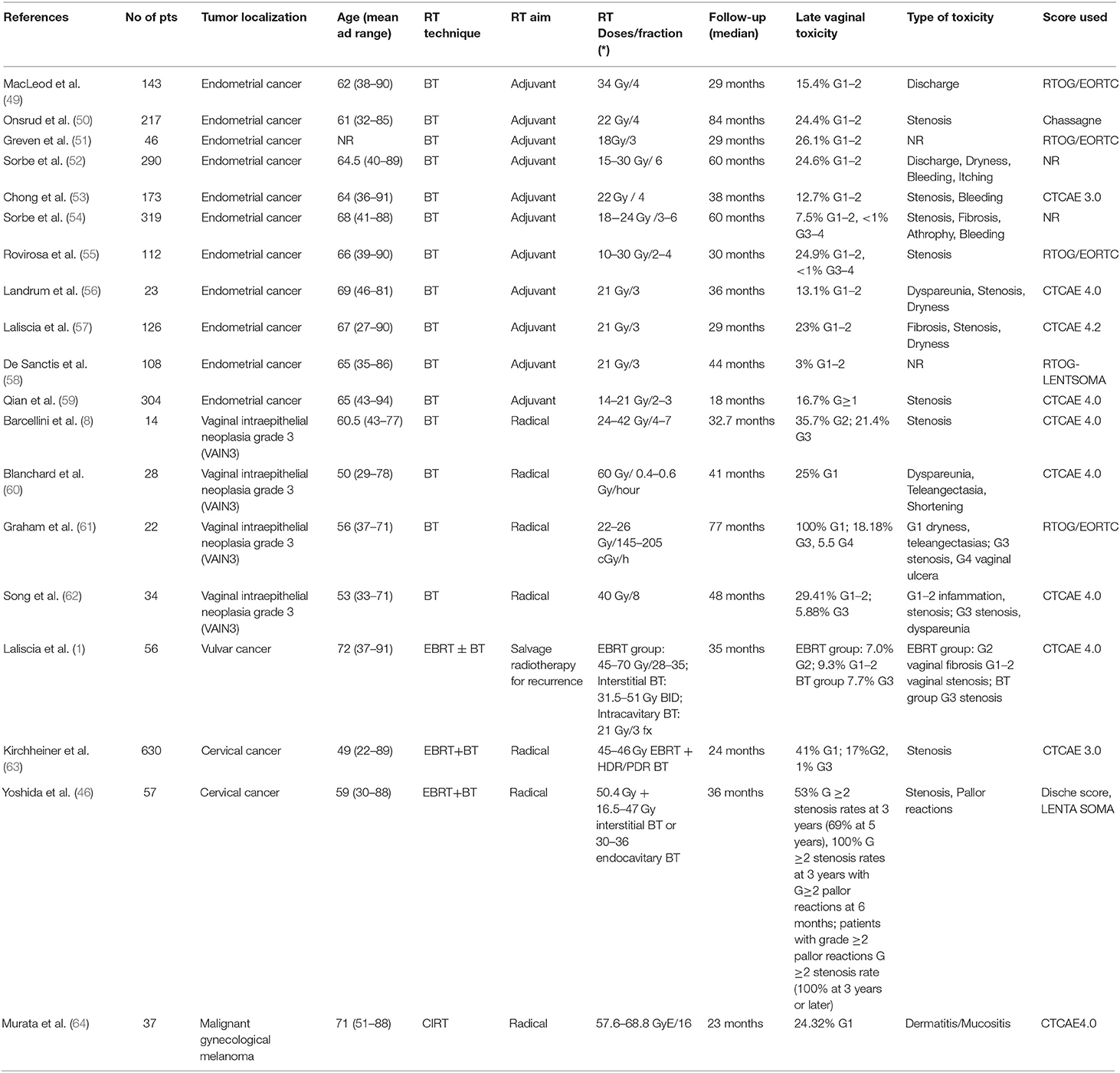
While the initial results led to 193 publications, after the screening process, the final sample included 19 studies. One prospective and 18 retrospective cohort studies were included, and the study characteristics are shown in Table 2. A total of 2,739 women were included in the studies, with 630 in the prospective cohort studies. The age of the patients across all studies ranged between 22 and 94 years. Endometrial and cervical cancers were the most frequently represented tumors. The majority of patients underwent BT alone (1,959 patients, 71.5%), followed by EBRT with or without a BT boost (743 patients, 27.13%) and a CIRT approach (37 women, 1.30%). The most common toxicity scale used was CTCAE (71), followed by RTOG/European Organization for Research and Treatment of Cancer (EORTC) (49, 51, 55, 61), RTOG/ Late Effects Normal Tissues (LENT)-Subjective, Objective, Management, Analytic (SOMA) (46, 58) (Table 2). In two studies, toxicities were scored by Dische (46) and Chassagne scores (72). The most common vaginal toxicities reported after pelvic RT (for each RT technique) were stenosis, dryness, and dyspareunia. In the articles analyzed, vulvodynia was not scored by the above-reported classifications.
Key Question 2: The Effectiveness of Physical Rehabilitation Including PFMT in the Management of Sexual Dysfunction After RT
With regards to GyC, five clinical trials (65–69) involving the role of PFMT were found, of which three were RCTs (Table 3). The number of participants ranged between 20 and 82. The duration of the intervention period ranged between 4 and 12 weeks, respectively, in the studies that reported the duration, frequency, and intensity of the training program. The interventions differed among studies and combined a plethora of modalities. The interventions ranged from supervised PFM strength and relaxation training combined with manual techniques (e.g., massage) to handling out a booklet with information or psychoeducational training. All the studies included vaginal device training. The one RCT reporting point estimates and variation between groups found that a multimodal PFMT intervention was statistically significantly superior to an untreated control group concerning PFM strength and sexual function (Table 3). The effectiveness of a psychoeducational approach seemed to depend on the age of participants as well as the dilation compliance (65). The PEDro score for the RCTs ranged between 4 and 5, for the analyzed studies (73) (Table 3).
Discussion
The present systematic review found that post-actinic vulvo-vaginal toxicity in long-term survivors from GyC is mostly represented by dryness, stenosis, and dyspareunia. In the analyzed data, the authors rarely suggested the management of this specific chronic toxicity, and it is also interesting to highlight that sexual health is poorly reported in these RT studies.
Regarding dosimetric studies, data related to vaginal and vulvar toxicities are scant (8, 63). Vulvar and vaginal tissues, currently vaguely included in the panel of OARs in RT planning, must be regarded due to the consequent morbidity (19).
Modern radiation techniques such as hadrontherapy are promising (5, 6, 74–76), but our review shows that data is scant both about dosimetric strategies to reduce vaginal toxicities and the radiation effect of PFM structures. These limitations highlight the need for further high-quality research.
The lack of data on RT and dosimetric studies is interesting considering that, in the PEDro database, with a combination of the following keywords “rehabilitation,” “pelvic floor muscle,” and “cancer,” we have found 24 records about rehabilitation in prostatic cancer, including one practice guidelines, seven systematic reviews, and 16 clinical trials. This is coherent with the results of the “igls-Vienna-sexmed-survey” (77) in which radiation oncologists showed higher awareness regarding male compared to female sexual functioning. Most radiation oncologists are not experts in treating sexual dysfunction (77), and more specific training seems of utmost importance to improve the attitudes and behavior toward sexual issues of GyC patients (78).
Usually, the main recommendations to women at the end of RT delivered to the pelvis with or without vaginal and/or endouterine BT are to resume sexual activity or to avoid the collapse of the vaginal walls with the use of vaginal dilators (79, 80). Unfortunately, this approach is often poorly tolerated by women with low adherence and compliance, often depending on the age of patients (65). Although in most studies the use of vaginal dilator was encouraged to reduce vaginal toxicity, Brennen et al. (81) reported a “very low” level of evidence of this approach, according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) analysis (82). Studies show that specific educational training also through a tailored-booklet proved to be effective in guaranteeing greater adherence and improving sexual health, especially in younger patients (65, 67, 68).
Data derived from trials based on men treated for prostate cancer in acute and long-term conditions revealed that there was no difference between pre- and post-radiation therapy in maximal thickness of external and internal anal sphincter (83–85). For men, RT toxicities after prostatic cancer are also reported to change the levator ani muscles and urogenital diaphragm (85) as well as reduce the ureteral length, and modifying periureteral muscles and the periprostatic portion of levator ani muscles due to post-RT muscle fibrosis of muscles (83).
In their systematic review and meta-analysis, Brennen et al. (78) reported that a combined approach, including PFMT, counseling, and physical exercises (core training and yoga), significantly improved the sexual health outcomes of GyC survivors. PFM functions, especially muscle strength, play a fundamental role in sexual function. Women with high levels of pelvic floor muscle contractions on physical evaluations achieved higher scores on Female Sexual Function Index (FSFI) (86), and the improvement of PFM control is related to a reduction of dyspareunia (18). However, the above-reported analysis included several pelvic health outcomes (i.e., bladder and rectal functioning) including women who did not undergo RT.
From a physical point of view, RT decreases the force of pelvic floor muscles with a reduction of recruitment of motor units. The modification in muscle tissue histology influences the competence to create strength and force rapidly, which may be important in counteracting the increased intra-abdominal pressure (87–90). The post-actinic muscle fiber damages could also lead to a reduction of spontaneous muscle activity and contractile response to stimulation (91) with a decrease in the ability to perform maximal strength rapidly (muscle power) as well as to maintain the same force in a series of repeated contractions (90, 92).
The rehabilitation programs aim to overcome the adverse effects of RT on pelvic floor muscles and to restore functionality in order to mitigate sexual distress, and bladder or bowel symptoms. The rehabilitation of women previously treated for pelvic cancers may decrease urinary incontinence and urgency, sexual dysfunction and discomfort, and improve the quality of life due to effective restoration of strength and an increase of blood flow in the pelvic floor tissue (66, 90, 93). However, our literature review found only 3 RCTs, and they had huge heterogeneity of populations, interventions, and use of outcome measures. The RCTs scored low to moderate on the PEDro score, with lack of blinding, loss to follow-up, and intention to treat analysis compromising the internal validity of the results. As the studies also included many different approaches to rehabilitation, it is not possible to conclude whether PFMT alone has a role in the rehabilitation of sexual function in GyC survivors.
In their RCT, Yang et al. (66) found that PFMT combined with core training improved pelvic strength and perceived sexual functioning leading to a significant increase in the proportion of sexually active women. However, whether core training has a role in the rehabilitation of PFMT has been debated, so far there is no evidence for this intervention alone or in combination with PFMT for urinary incontinence (94, 95) or sexual dysfunction (96).
The potential preventive role of routine use of vaginal dilators and the level of evidence about this practice after RT are still not clear (79, 81). A Cochrane review (79) reported no dependable evidence to consider that routine and constant use of vaginal dilators during RT prevents vaginal stenosis, although this practice is associated with lower rates of self-reported stenosis. The GRADE analysis by Brennen et al. (81) showed a “very low” level of evidence for the decrease in vaginal complications with the high use of vaginal dilators.
A multimodal approach with PFMT and the use of vaginal moisturizers seem feasible for GyC survivors (69). Hyaluronic acid seems to be effective and safe in the treatment of vaginal acute and late RT toxicities (97). Despite the controversial carcinogenesis risk of hormone replacement therapy and the lack of high-level evidence (98) in their systematic review, Vargiu et al. reported the benefits of this approach for the management of early menopause in patients with cervical cancer (16).
Conclusion
Due to few data, large heterogeneity, and the low methodological quality of the included studies in our review, results should be interpreted with caution; however, our findings indicate that the rehabilitation approach (including PFMT and vaginal dilator training) may be effective and feasible in improving sexual function and in GyC patients who have undergone RT. To improve our knowledge and evidence for clinical practice of GyC survivors, we suggest a multidisciplinary approach between oncologists (radiation oncologist, medical oncologist, gynecological oncologists) and experts in rehabilitation and physical therapy in addressing the following research questions:
• What is the effect of RT with or without BT on the female PFM?
• What is the effect of PFMT in women with post-RT vulvo-vaginal toxicity?
• Does the effect of PFMT on vulvo-vaginal symptoms differ according to the total RT dose, fractionation schedule, and type of RT?
• Does preventive PFMT, before and during RT, improve sexual functioning?
• Is PFMT cost-effective in GyC patients?
Large, well-designed RCTs with long-term follow-up, which explicitly measure adherence and investigate the effect on vaginal function are needed to answer these questions.
Author Contributions
AB and KB contributed to the concept and research design. AB, MD, and SV contributed to data collection. AB and MD contributed to the writing. HB, FD, EO, and BG critically revised the manuscript. All the authors read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Laliscia C, Gadducci A, Fabrini MG, Barcellini A, Parietti E, Pasqualetti F, et al. Definitive radiotherapy for recurrent vulvar carcinoma after primary surgery: a two-institutional Italian experience. Tumori. (2019) 105:225–30. doi: 10.1177/0300891618811279
2. Laliscia C, Fabrini MG, Cafaro I, Barcellini A, Baldaccini D, Miniati M, et al. Adjuvant radiotherapy in high-risk squamous cell carcinoma of the vulva: a two-Institutional Italian Experience. Oncol Res Treat. (2017) 40:778–83. doi: 10.1159/000479876
3. Laliscia C, Gadducci A, Fabrini MG, Barcellini A, Guerrieri ME, Parietti E, et al. Definitive radiotherapy for primary squamous cell carcinoma of the vagina: are high-dose external beam radiotherapy and high-dose-rate brachytherapy boost the best treatment? experience of two Italian Institutes. Oncol Res Treat. (2017) 40:652–3. doi: 10.1159/000480350
4. Lokich E. Gynecologic cancer survivorship. Obstet Gynecol Clin North Am. (2019) 46:165–78. doi: 10.1016/j.ogc.2018.10.002
5. Barcellini A, Vitolo V, Mastella E, Mirandola A, Valvo F. Letter to the Editor concerning “Re-irradiation in gynaecological cancers, present experiences and future hopes.” J Radiat Oncol. (2019) 8:355–6. doi: 10.1007/s13566-019-00396-w
6. Sadozye AH. Re-irradiation in gynaecological malignancies: a review. Clin Oncol. (2018) 30:110–5. doi: 10.1016/j.clon.2017.11.013
7. Stinesen Kollberg K, Waldenström A-C, Bergmark K, Dunberger G, Rossander A, Wilderäng U, et al. Reduced vaginal elasticity, reduced lubrication, and deep and superficial dyspareunia in irradiated gynecological cancer survivors. Acta Oncol. (2015) 54:772–9. doi: 10.3109/0284186X.2014.1001036
8. Barcellini A, Lecchi M, Tenconi C, Macciotta A, Pignoli E, Pappalardi B, et al. High-dose-rate brachytherapy for high-grade vaginal intraepithelial neoplasia: a dosimetric analysis. J Contemp Brachyther. (2019) 11:146–51. doi: 10.5114/jcb.2019.84696
9. Delishaj D, Barcellini A, D'Amico R, Ursino S, Pasqualetti F, Fumagalli IC, et al. Vaginal toxicity after high-dose-rate endovaginal brachytherapy: 20 years of results. J Contemp Brachyther. (2018) 10:559–66. doi: 10.5114/jcb.2018.79713
10. Dunberger G, Lind H, Steineck G, Waldenström A-C, Nyberg T, al-Abany M, et al. Fecal incontinence affecting quality of life and social functioning among long-term gynecological cancer survivors. Int J Gynecol cancer Off J Int Gynecol Cancer Soc. (2010) 20:449–60. doi: 10.1111/IGC.0b013e3181d373bf
11. Dunberger G, Lindquist H, Waldenström A-C, Nyberg T, Steineck G, Åvall-Lundqvist E. Lower limb lymphedema in gynecological cancer survivors–effect on daily life functioning. Support care cancer. Off J Multinatl Assoc Support Care Cancer. (2013) 21:3063–70. doi: 10.1007/s00520-013-1879-3
12. Smet S, Pötter R, Haie-Meder C, Lindegaard JC, Schulz-Juergenliemk I, Mahantshetty U, et al. Fatigue, insomnia and hot flashes after definitive radiochemotherapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: an analysis from the EMBRACE study. Radiother Oncol J Eur Soc Ther Radiol Oncol. (2018) 127:440–8. doi: 10.1016/j.radonc.2018.03.009
13. Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol Off J Eur Soc Med Oncol. (2020) 31:713–23. doi: 10.1016/j.annonc.2020.02.016
14. Ferrandina G, Petrillo M, Mantegna G, Fuoco G, Terzano S, Venditti L, et al. Evaluation of quality of life and emotional distress in endometrial cancer patients: a 2-year prospective, longitudinal study. Gynecol Oncol. (2014) 133:518–25. doi: 10.1016/j.ygyno.2014.03.015
15. Cianci S, Rosati A, Capozzi VA, Tarascio M, Uccella S, Palumbo M, et al. Quality of life and sexual functioning of patient affected by endometrial cancer. Minerva Med. (2021) 112:81–95. doi: 10.23736/S0026-4806.20.07081-0
16. Vargiu V, Amar ID, Rosati A, Dinoi G, Turco LC, Capozzi VA, et al. Hormone replacement therapy and cervical cancer: a systematic review of the literature. Climacteric. (2021) 24:120–7. doi: 10.1080/13697137.2020.1826426
17. Cianci S, Tarascio M, Rosati A, Caruso S, Uccella S, Cosentino F, et al. Sexual function and quality of life of patients affected by ovarian cancer. Minerva Med. (2019) 110:320–9. doi: 10.23736/S0026-4806.19.06080-4
18. Huffman LB, Hartenbach EM, Carter J, Rash JK, Kushner DM. Maintaining sexual health throughout gynecologic cancer survivorship: A comprehensive review and clinical guide. Gynecol Oncol. (2016) 140:359–68. doi: 10.1016/j.ygyno.2015.11.010
19. Kirchheiner K, Fidarova E, Nout RA, Schmid MP, Sturdza A, Wiebe E, et al. Radiation-induced morphological changes in the vagina. Strahlentherapie und Onkol. (2012) 188:1010–9. doi: 10.1007/s00066-012-0222-0
20. Katz A. Interventions for sexuality after pelvic radiation therapy and gynecological cancer. Cancer J. (2009) 15:45–7. doi: 10.1097/PPO.0b013e31819585cf
21. Ayling K, Ussher JM. “If sex hurts, am i still a woman?” the subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. (2008) 37:294–304. doi: 10.1007/s10508-007-9204-1
22. Desrochers G, Bergeron S, Landry T, Jodoin M. Do psychosexual factors play a role in the etiology of provoked vestibulodynia? A critical review. J Sex Marital Ther. (2008) 34:198–226. doi: 10.1080/00926230701866083
23. Bergeron S, Rosen NO, Morin M. Genital pain in women: beyond interference with intercourse. Pain. (2011) 152:1223–5. doi: 10.1016/j.pain.2011.01.035
24. Hazewinkel MH, Sprangers MAG, van der Velden J, van der Vaart CH, Stalpers LJA, Burger MPM, et al. Long-term cervical cancer survivors suffer from pelvic floor symptoms: a cross-sectional matched cohort study. Gynecol Oncol. (2010) 117:281–6. doi: 10.1016/j.ygyno.2010.01.034
25. Greear G, Lefkowits C, Parrillo LM, Flynn BJ. Incontinence, voiding dysfunction, and other urologic complications after radiotherapy for gynecologic malignancies. Curr Bladder Dysfunct Rep. (2016) 11:88–97. doi: 10.1007/s11884-016-0354-7
26. Zomkowski K, Toryi AM, Sacomori C, Dias M, Sperandio FF. Sexual function and quality of life in gynecological cancer pre- and post-short-term brachytherapy: a prospective study. Arch Gynecol Obstet. (2016) 294:833–40. doi: 10.1007/s00404-016-4099-5
27. Bernard S, Ouellet M-P, Moffet H, Roy J-S, Dumoulin C. Effects of radiation therapy on the structure and function of the pelvic floor muscles of patients with cancer in the pelvic area: a systematic review. J Cancer Surviv. (2016) 10:351–62. doi: 10.1007/s11764-015-0481-8
28. Bednarova R, Biancuzzi H, Rizzardo A, Dal Mas F, Massaro M, Cobianchi L, et al. Cancer rehabilitation and physical activity: the ‘Oncology in Motion' project. J Cancer Educ. (2020) 20:1920. doi: 10.1007/s13187-020-01920-0
29. Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. (2012) 62:243–74. doi: 10.3322/caac.21142
30. Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. (2019) 51:2375–90. doi: 10.1249/MSS.0000000000002116
31. Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW, et al. framework for prescription in exercise-oncology research. J Cachexia Sarcopenia Muscle. (2015) 6:115–24. doi: 10.1002/jcsm.12042
32. Galvão DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. (2005) 23:899–909. doi: 10.1200/JCO.2005.06.085
33. Puetz TW, Herring MP. Differential effects of exercise on cancer-related fatigue during and following treatment: a meta-analysis. Am J Prev Med. (2012) 43:e1–24. doi: 10.1016/j.amepre.2012.04.027
34. Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev Publ Am Assoc Cancer Res Am Soc Prev Oncol. (2011) 20:123–33. doi: 10.1158/1055-9965.EPI-10-0988
35. Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochr Database Syst Rev. (2012) 2012:CD007566. doi: 10.1002/14651858.CD007566.pub2
36. van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol Off J Am Soc Clin Oncol. (2015) 33:1918–27. doi: 10.1200/JCO.2014.59.1081
37. Vanlerenberghe E, Sedda A-L, Ait-Kaci F. The impact of gynaecological cancers on woman's sexuality and her couple. Bull Cancer. (2015) 102:454–62. doi: 10.1016/j.bulcan.2015.02.008
38. Manne SL, Kashy DA, Virtue S, Criswell KR, Kissane DW, Ozga M, et al. Acceptance, social support, benefit-finding, and depression in women with gynecological cancer. Qual Life Res Int J Qual life Asp Treat Care Rehabil. (2018) 27:2991–3002. doi: 10.1007/s11136-018-1953-x
39. Del Pup L, Villa P, Amar ID, Bottoni C, Scambia G. Approach to sexual dysfunction in women with cancer. Int J Gynecol cancer Off J Int Gynecol Cancer Soc. (2019) 29:630–4. doi: 10.1136/ijgc-2018-000096
40. Massaro M, Dumay J, Guthrie J. On the shoulders of giants: undertaking a structured literature review in accounting. Account Audit Account J. (2016) 29:767–801. doi: 10.1108/AAAJ-01-2015-1939
41. Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S, PICO. PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. (2014) 14:579. doi: 10.1186/s12913-014-0579-0
42. Ayeleke RO, Hay-Smith EJC, Omar MI. Pelvic floor muscle training added to another active treatment versus the same active treatment alone for urinary incontinence in women. Cochr Database Syst Rev. (2015) 2015:CD010551. doi: 10.1002/14651858.CD010551.pub3
43. Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochr Database Syst Rev. (2018) 10:CD005654. doi: 10.1002/14651858.CD005654.pub4
44. CTCAE v 5.0. Available online at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
45. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. (1995) 31:1341–6. doi: 10.1016/0360-3016(95)00060-C
46. Yoshida K, Yamazaki H, Nakamura S, Masui K, Kotsuma T, Akiyama H, et al. Role of vaginal pallor reaction in predicting late vaginal stenosis after high-dose-rate brachytherapy in treatment-naive patients with cervical cancer. J Gynecol Oncol. (2015) 26:179–84. doi: 10.3802/jgo.2015.26.3.179
47. Bornstein J, Goldstein AT, Stockdale CK, Bergeron S, Pukall C, Zolnoun D, et al. 2015 ISSVD, ISSWSH, and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. J Sex Med. (2016) 13:607–12. doi: 10.1016/j.jsxm.2016.02.167
48. Moher D, Liberati A, Tetzlaff J, Altman DG, The The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
49. MacLeod C, Fowler A, Duval P, D'Costa I, Dalrymple C, Firth I, et al. High-dose-rate brachytherapy alone post-hysterectomy for endometrial cancer. Int J Radiat Oncol Biol Phys. (1998) 42:1033–9. doi: 10.1016/S0360-3016(98)00292-2
50. Onsrud M, Strickert T, Marthinsen AB. Late reactions after postoperative high-dose-rate intravaginal brachytherapy for endometrial cancer: a comparison of standardized and individualized target volumes. Int J Radiat Oncol Biol Phys. (2001) 49:749–55. doi: 10.1016/s0360-3016(00)01464-4
51. Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Preliminary analysis of RTOG 9708: Adjuvant postoperative radiotherapy combined with cisplatin/paclitaxel chemotherapy after surgery for patients with high-risk endometrial cancer. Int J Radiat Oncol Biol Phys. (2004) 59:168–73. doi: 10.1016/j.ijrobp.2003.10.019
52. Sorbe B, Straumits A, Karlsson L. Intravaginal high-dose-rate brachytherapy for stage I endometrial cancer: a randomized study of two dose-per-fraction levels. Int J Radiat Oncol Biol Phys. (2005) 62:1385–9. doi: 10.1016/j.ijrobp.2004.12.079
53. Chong I, Hoskin PJ. Vaginal vault brachytherapy as sole postoperative treatment for low-risk endometrial cancer. Brachytherapy. (2008) 7:195–9. doi: 10.1016/j.brachy.2008.01.001
54. Sorbe B, Nordström B, Mäenpaa¨ J, Kuhelj J, Kujelj D, Okkan S, et al. Intravaginal brachytherapy in FIGO stage I low-risk endometrial cancer: a controlled randomized study. Int J Gynecol Cancer. (2009) 19:873–8. doi: 10.1111/IGC.0b013e3181a6c9df
55. Rovirosa A, Valduvieco I, Ascaso C, Herreros A, Bautista C, Romera I, et al. Daily schedule for high-dose-rate brachytherapy in postoperative treatment of endometrial carcinoma. Clin Transl Oncol Off Publ Fed Spanish Oncol Soc Natl Cancer Inst Mex. (2013) 15:111–6. doi: 10.1007/s12094-012-0893-0
56. Landrum LM, Nugent EK, Zuna RE, Syzek E, Mannel RS, Moore KN, et al. Phase II trial of vaginal cuff brachytherapy followed by chemotherapy in early stage endometrial cancer patients with high-intermediate risk factors. Gynecol Oncol. (2014) 132:50–4. doi: 10.1016/j.ygyno.2013.11.005
57. Laliscia C, Delishaj D, Fabrini MG, Gonneli A, Morganti R, Perrone F, et al. Acute and late vaginal toxicity after adjuvant high-dose-rate vaginal brachytherapy in patients with intermediate risk endometrial cancer: is local therapy with hyaluronic acid of clinical benefit? J Contemp Brachytherapy. (2016) 8:512–7. doi: 10.5114/jcb.2016.64511
58. De Sanctis V, Musio D, De Felice F, Marampon F, Valeriani M, Bonome P, et al. One-week vaginal brachytherapy schedule as exclusive adjuvant post-operative treatment in intermediate- and high-intermediate-risk endometrial cancer patients. J Contemp Brachyther. (2020) 12:124–30. doi: 10.5114/jcb.2020.94581
59. Qian JM, Stahl JM, Young MR, Ratner E, Damast S. Impact of vaginal cylinder diameter on outcomes following brachytherapy for early stage endometrial cancer. J Gynecol Oncol. (2017) 28:e84. doi: 10.3802/jgo.2017.28.e84
60. Blanchard P, Monnier L, Dumas I, Morice P, Pautier P, Duvillard P, et al. Low-dose-rate definitive brachytherapy for high-grade vaginal intraepithelial neoplasia. Oncologist. (2011) 16:182–8. doi: 10.1634/theoncologist.2010-0326
61. Graham K, Wright K, Cadwallader B, Reed NS, Symonds RP. 20-year retrospective review of medium dose rate intracavitary brachytherapy in VAIN3. Gynecol Oncol. (2007) 106:105–11. doi: 10.1016/j.ygyno.2007.03.005
62. Song JH, Lee JH, Lee JH, Park JS, Hong SH, Jang HS, et al. High-dose-rate brachytherapy for the treatment of vaginal intraepithelial neoplasia. Cancer Res Treat. (2014) 46:74–80. doi: 10.4143/crt.2014.46.1.74
63. Kirchheiner K, Nout RA, Lindegaard JC, Haie-Meder C, Mahantshetty U, Segedin B, et al. Dose-effect relationship and risk factors for vaginal stenosis after definitive radio(chemo)therapy with image-guided brachytherapy for locally advanced cervical cancer in the EMBRACE study. Radiother Oncol J Eur Soc Ther Radiol Oncol. (2016) 118:160–6. doi: 10.1016/j.radonc.2015.12.025
64. Murata H, Okonogi N, Wakatsuki M, Kato S, Kiyohara H, Karasawa K, et al. Long-Term Outcomes of Carbon-Ion Radiotherapy for Malignant Gynecological Melanoma. Cancers (Basel). (2019) 11:482. doi: 10.3390/cancers11040482
65. Robinson JW, Faris PD, Scott CB. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynecological carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys. (1999) 44:497–506. doi: 10.1016/S0360-3016(99)00048-6
66. Yang EJ, Lim J-Y, Rah UW, Kim YB. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol. (2012) 125:705–11. doi: 10.1016/j.ygyno.2012.03.045
67. Bakker RM, Mens JWM, de Groot HE, Tuijnman-Raasveld CC, Braat C, Hompus WCP, et al. A nurse-led sexual rehabilitation intervention after radiotherapy for gynecological cancer. Support Care Cancer. (2017) 25:729–37. doi: 10.1007/s00520-016-3453-2
68. Lubotzky FP, Butow P, Hunt C, Costa DSJ, Laidsaar-Powell R, Carroll S, et al. A psychosexual rehabilitation booklet increases vaginal dilator adherence and knowledge in women undergoing pelvic radiation therapy for gynaecological or anorectal cancer: a randomised controlled trial. Clin Oncol. (2019) 31:124–31. doi: 10.1016/j.clon.2018.11.035
69. Cyr M-P, Dumoulin C, Bessette P, Pina A, Gotlieb WH, Lapointe-Milot K, et al. Feasibility, acceptability and effects of multimodal pelvic floor physical therapy for gynecological cancer survivors suffering from painful sexual intercourse: a multicenter prospective interventional study. Gynecol Oncol. (2020) 192–201. doi: 10.1016/j.physio.2021.09.003
70. Physiotherapy Evidence Database. PEDro Scale. The Centre of Evidence-Based Physiotherapy. Sydney: University of Sydney.
71. CTCAE v. 4.0. Available online at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40 (accessed November 10, 2021).
72. Chassagne D, Sismondi P, Horiot JC, Sinistrero G, Bey P, Zola P, et al. A glossary for reporting complications of treatment in gynecological cancers. Radiother Oncol J Eur Soc Ther Radiol Oncol. (1993) 26:195–202. doi: 10.1016/0167-8140(93)90260-F
73. de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. (2009) 55:129–33. doi: 10.1016/S0004-9514(09)70043-1
74. Barcellini A, Gadducci A, Laliscia C, Imparato S, Vitolo V, Preda L, et al. Adenoid cystic carcinoma of Bartholin's gland: what is the best approach? Oncology. (2020) 2020:1–7. doi: 10.1159/000506485
75. Barcellini A, Vitolo V, Facoetti A, Fossati P, Preda L, Fiore MR, Vischioni B, et al. Feasibility of carbon ion radiotherapy in the treatment of gynecological melanoma. In vivo. (2019) 33:11497. doi: 10.21873/invivo.11497
76. Barcellini A, Roccio M, Laliscia C, Zanellini F, Pettinato D, Valvo F, et al. Endometrial cancer: when upfront surgery is not an option. Oncology. (2020) 99:65–71. doi: 10.1159/000510690
77. Bräutigam E, Schratter-Sehn A, Kottmel A, Bitzer J, Teleky B, Ucsnik L. Do radiation oncologists talk about sexual health and dysfunction with their cancer patients? Results of the igls-vienna-sexmed-survey. Clin Transl Radiat Oncol. (2020) 21:120–6. doi: 10.1016/j.ctro.2020.01.005
78. Wang J, Sun X, Cai R, Jiao S, Yu H, Zhang Y, et al. Attitudes and behavior of radiation oncologists toward sexual issues of cervical cancer patients who receive radiation therapy: a survey in China. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. (2013) 23:393–8. doi: 10.1097/IGC.0b013e31828080ee
79. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochr Database Syst Rev. (2014) 2014:CD007291. doi: 10.1002/14651858.CD007291.pub3
80. Damast S, Alektiar KM, Goldfarb S, Eaton A, Patil S, Mosenkis J, et al. Sexual functioning among endometrial cancer patients treated with adjuvant high-dose-rate intra-vaginal radiation therapy. Int J Radiat Oncol Biol Phys. (2012) 84:e187–93. doi: 10.1016/j.ijrobp.2012.03.030
81. Brennen R, Lin K-Y, Denehy L, Frawley HC. The effect of pelvic floor muscle interventions on pelvic floor dysfunction after gynecological cancer treatment: a systematic review. Phys Ther. (2020) 100:1357–71. doi: 10.1093/ptj/pzaa081
82. Schunemann H, Brozek J, Guyatt G, Oxman A eds. Grading of Recommendations, Assessment, Development Evaluation (GRADE) Working Group. Handbook for grading the quality of evidence the strength of recommendations using the GRADE approach. The Cochrane Collaboration (2013). Available online at: https://gdt.gradepro.org/app/handbook/handbook.html (accessed November 10, 2021).
83. Marigliano C, Donati OF, Vargas HA, Akin O, Goldman DA, Eastham JA, et al. MRI findings of radiation-induced changes in the urethra and periurethral tissues after treatment for prostate cancer. Eur J Radiol. (2013) 82:e775–81. doi: 10.1016/j.ejrad.2013.09.011
84. Yeoh EE, Botten R, Russo A, McGowan R, Fraser R, Roos D, et al. Chronic effects of therapeutic irradiation for localized prostatic carcinoma on anorectal function. Int J Radiat Oncol Biol Phys. (2000) 47:915–24. doi: 10.1016/S0360-3016(00)00487-9
85. Coakley FV, Hricak H, Wefer AE, Speight JL, Kurhanewicz J, Roach M. Brachytherapy for prostate cancer: endorectal MR imaging of local treatment-related changes. Radiology. (2001) 219:817–21. doi: 10.1148/radiology.219.3.r01jn46817
86. Lowenstein L, Gruenwald I, Gartman I, Vardi Y. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. (2010) 21:553–6. doi: 10.1007/s00192-009-1077-5
87. Birder L, de Groat W, Mills I, Morrison J, Thor K, Drake M. Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn. (2010) 29:128–39. doi: 10.1002/nau.20837
88. Ashton-Miller JA, DeLancey JOL. Functional anatomy of the female pelvic floor. Ann N Y Acad Sci. (2007) 1101:266–96. doi: 10.1196/annals.1389.034
89. Folland JP, Buckthorpe MW, Hannah R. Human capacity for explosive force production: neural and contractile determinants. Scand J Med Sci Sports. (2014) 24:894–906. doi: 10.1111/sms.12131
90. Bernard S, Moffet H, Plante M, Ouellet M-P, Leblond J, Dumoulin C. Pelvic-floor properties in women reporting urinary incontinence after surgery and radiotherapy for endometrial cancer. Phys Ther. (2017) 97:438–48. doi: 10.1093/ptj/pzx012
91. Lorenzi B, Brading AF, Martellucci J, Cetta F, Mortensen NJMC. Short-term effects of neoadjuvant chemoradiotherapy on internal anal sphincter function: a human in vitro study. Dis Colon Rectum. (2012) 55:465–72. doi: 10.1097/DCR.0b013e31824154a0
92. Lovegrove Jones RC, Peng Q, Stokes M, Humphrey VF, Payne C, Constantinou CE. Mechanisms of pelvic floor muscle function and the effect on the urethra during a cough. Eur Urol. (2010) 57:1101–10. doi: 10.1016/j.eururo.2009.06.011
93. Sacomori C, Araya-Castro P, Diaz-Guerrero P, Ferrada IA, Martínez-Varas AC, Zomkowski K. Pre-rehabilitation of the pelvic floor before radiation therapy for cervical cancer: a pilot study. Int Urogynecol J. (2020) 31:2411–8. doi: 10.1007/s00192-020-04391-5
94. Bø K, Herbert RD. There is not yet strong evidence that exercise regimens other than pelvic floor muscle training can reduce stress urinary incontinence in women: a systematic review. J Physiother. (2013) 59:159–68. doi: 10.1016/S1836-9553(13)70180-2
95. Bø K, Mørkved S, Frawley H, Sherburn M. Evidence for benefit of transversus abdominis training alone or in combination with pelvic floor muscle training to treat female urinary incontinence: a systematic review. Neurourol Urodyn. (2009) 28:368–73. doi: 10.1002/nau.20700
96. Ferreira CHJ, Dwyer PL, Davidson M, De Souza A, Ugarte JA, Frawley HC. Does pelvic floor muscle training improve female sexual function? A systematic review. Int Urogynecol J. (2015) 26:1735–50. doi: 10.1007/s00192-015-2749-y
97. Cosentino D, Piro F. Hyaluronic acid for treatment of the radiation therapy side effects: a systematic review. Eur Rev Med Pharmacol Sci. (2018) 22:7562–72. doi: 10.26355/eurrev_201811_16298
Keywords: gynecological cancers, pelvic floor muscle training, radiotherapy, rehabilitation, sexual health, vaginal toxicity
Citation: Barcellini A, Dominoni M, Dal Mas F, Biancuzzi H, Venturini SC, Gardella B, Orlandi E and Bø K (2022) Sexual Health Dysfunction After Radiotherapy for Gynecological Cancer: Role of Physical Rehabilitation Including Pelvic Floor Muscle Training. Front. Med. 8:813352. doi: 10.3389/fmed.2021.813352
Received: 11 November 2021; Accepted: 09 December 2021;
Published: 03 February 2022.
Edited by:
Andrea Rosati, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Baroni Alessandro, Agostino Gemelli University Polyclinic (IRCCS), ItalyCopyright © 2022 Barcellini, Dominoni, Dal Mas, Biancuzzi, Venturini, Gardella, Orlandi and Bø. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amelia Barcellini, amelia.barcellini@cnao.it
†These authors share first authorship
‡These authors share last authorship
 Amelia Barcellini1*†
Amelia Barcellini1*†  Mattia Dominoni
Mattia Dominoni Francesca Dal Mas
Francesca Dal Mas Ester Orlandi
Ester Orlandi