CO Preferential Photo-Oxidation in Excess of Hydrogen in Dark and Simulated Solar Light Irradiation over AuCu-Based Catalysts on SBA-15 Mesoporous Silica-Titania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SBA and Ti-SBA
2.2. Preparation of Au and AuCu Supported Nanoparticles
2.3. Characterization of Catalysts
2.4. Photocatalytic Activity in CO-PROX
3. Results
3.1. Characterization of Supports
3.2. Characterization of Photocatalysts
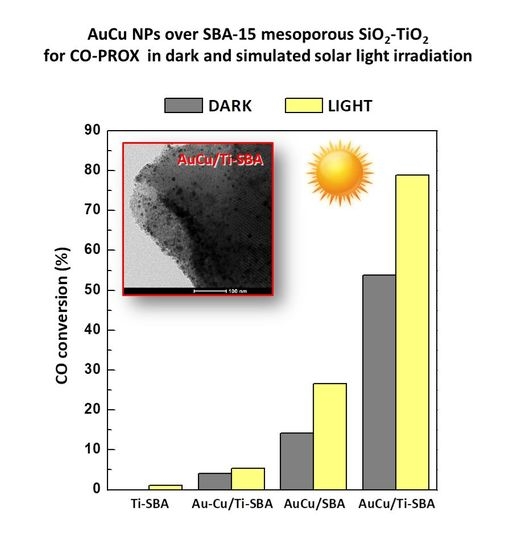
3.3. Photocatalytic Activity in Preferential Oxidation of CO (CO-PROX) Reaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Murcia, J.J.; Hernández-Laverde, M.; Rojas, H.; Muñoz, E.; Navío, J.A.; Hidalgo, M.C. Study of the effectiveness of the flocculation-photocatalysis in the treatment of wastewater coming from dairy industries. J. Photochem. Photobiol. A Chem. 2018, 358, 256–264. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.N.; Bard, A.J. Heterogeneous photocatalytic oxidation of cyanide ion in aqueous solutions at titanium dioxide powder. J. Am. Chem. Soc. 1977, 99, 303–304. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, X.-D.; Chang, C.-T. Photocatalytic degradation of tetracycline by Ti-MCM-41 prepared at room temperature and biotoxicity of degradation products. Appl. Surf. Sci. 2017, 416, 248–258. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Xu, Y.-J. Heterogeneous Photocatalysis: From Fundamentals to Green Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Albero, J.; García, H. Photocatalytic CO2 Reduction; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–31. [Google Scholar]
- Gadgil, T.; Ibrayev, N.; Nuraje, N. Photocatalytic Water Oxidation; Springer: Berlin/Heidelberg, Germany, 2016; pp. 33–61. [Google Scholar]
- García-López, E.I.; Marcì, G.; Palmisano, L. Heteropolyacid-Based Heterogeneous Photocatalysts for Environmental Application; Springer: Berlin/Heidelberg, Germany, 2016; pp. 63–107. [Google Scholar]
- Xiao, F.-X.; Liu, B. 1D TiO2 Nanotube-Based Photocatalysts; Springer: Berlin/Heidelberg, Germany, 2016; pp. 151–173. [Google Scholar]
- Li, X.; Yu, J. Water Splitting by Photocatalytic Reduction; Springer: Berlin/Heidelberg, Germany, 2016; pp. 175–210. [Google Scholar]
- Yuan, L.; Zhang, N.; Xu, Y.-J.; Colmenares, J.C. Solar–Chemical Energy Conversion by Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2016; pp. 249–282. [Google Scholar]
- Wisitsoraat, A.; Tuantranont, A.; Comini, E.; Sberveglieri, G.; Wlodarski, W. Characterization of n-type and p-type semiconductor gas sensors based on NiOx doped TiO2 thin films. Thin Solid Films 2009, 517, 2775–2780. [Google Scholar] [CrossRef]
- Nolan, N.T.; Seery, M.K.; Pillai, S.C. Spectroscopic Investigation of the Anatase-to-Rutile Transformation of Sol−Gel-Synthesized TiO2 Photocatalysts. J. Phys. Chem. C 2009, 113, 16151–16157. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.-C.; Zakaria, R.; Ying, J.Y. Role of Particle Size in Nanocrystalline TiO2-Based Photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Gude, K.; Gun’ko, V.M.; Blitz, J.P. Adsorption and photocatalytic decomposition of methylene blue on surface modified silica and silica-titania. Colloids Surf. A Physicochem. Eng. Asp. 2008, 325, 17–20. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Asahi, R.; Taga, Y.; Mannstadt, W.; Freeman, A.J. Electronic and optical properties of anatase TiO2. Phys. Rev. B 2000, 61, 7459–7465. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Notari, B. Microporous Crystalline Titanium Silicates. Adv. Catal. 1996, 41, 253–334. [Google Scholar] [CrossRef]
- Takayoshi, S.; Koizumi, N.; Hatakeyama, K.; Ikeuchi, T. Post-synthesis of TiO2 Dispersed inside the Pore Channels of SBA-15 and its Photocatalytic Activity for the Degradation of Methylene Blue. Int. J. Soc. Mater. Eng. Resour. 2011, 18, 11–17. [Google Scholar]
- Wang, J.; Ge, H.; Bao, W. Synthesis and characteristics of SBA-15 with thick pore wall and high hydrothermal stability. Mater. Lett. 2015, 145, 312–315. [Google Scholar] [CrossRef]
- Lin, H.-P.; Tang, C.-Y.; Lin, C.-Y. Detailed Structural Characterizations of SBA-15 and MCM-41 Mesoporous Silicas on a High-Resolution Transmission Electron Microscope. J. Chin. Chem. Soc. 2002, 49, 981–988. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Qian, E.W. Synthesis of mesoporous Ti-inserted SBA-15 and CoMo/Ti-SBA-15 catalyst for hydrodesulfurization and hydrodearomatization. Microporous Mesoporous Mater. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Bharat, L.N.; Johnson, O.; Komarneni, S. Direct Synthesis of Titanium-Substituted Mesoporous SBA-15 Molecular Sieve under Microwave−Hydrothermal Conditions. Chem. Mater. 2001, 13, 552–557. [Google Scholar] [CrossRef]
- Jung, W.Y.; Baek, S.H.; Yang, J.S.; Lim, K.-T.; Lee, M.S.; Lee, G.-D.; Park, S.S.; Hong, S.-S. Synthesis of Ti-containing SBA-15 materials and studies on their photocatalytic decomposition of orange II. Catal. Today 2008, 131, 437–443. [Google Scholar] [CrossRef]
- Chen, L.Y.; Chuah, G.K.; Jaenicke, S. Ti-containing MCM-41 catalysts for liquid phase oxidation of cyclohexene with aqueous H2O2 and tert-butyl hydroperoxide. Catal. Lett. 1998, 50, 107–114. [Google Scholar] [CrossRef]
- Amezcua, J.C.; Lizama, L.; Salcedo, C.; Puente, I.; Domínguez, J.M.; Klimova, T. NiMo catalysts supported on titania-modified SBA-16 for 4,6-dimethyldibenzothiophene hydrodesulfurization. Catal. Today 2005, 107–108, 578–588. [Google Scholar] [CrossRef]
- Sarina, S.; Waclawik, E.R.; Zhu, H. Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chem. 2013, 15, 1814. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q. Photochromism into nanosystems: Towards lighting up the future. Chem. Soc. Rev. 1044, 47, 1044–1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Astruc, D. Nanogold plasmonic photocatalysis for organic synthesis and clean energy conversion. Chem. Soc. Rev. 2014, 43, 7188–7216. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, W.; Wang, J.; Yang, Q.; Hou, Q.; Ju, M.; Jiao, T.; Wei, D.; Song, X.; Sun, T.; et al. Gold nanoparticle-modified TiO2/SBA-15 nanocomposites as active plasmonic photocatalysts for the selective oxidation of aromatic alcohols. RSC Adv. 2016, 6, 70352–70363. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, W.; Lee, S.; Gao, J. Photocatalytic performance of plasma sprayed Pt-modified TiO2 coatings under visible light irradiation. Catal. Commun. 2007, 8, 906–912. [Google Scholar] [CrossRef]
- Lv, J.; Zhu, Q.; Zeng, Z.; Zhang, M.; Yang, J.; Zhao, M.; Wang, W.; Cheng, Y.; He, G.; Sun, Z. Enhanced photocurrent and photocatalytic properties of porous ZnO thin film by Ag nanoparticles. J. Phys. Chem. Solids 2017, 111, 104–109. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Li, W.; Ju, M. Microwave-assisted hydrothermal synthesis of Au/TiO2/SBA-15 for enhanced visible-light photoactivity. Mater. Lett. 2015, 159, 131–134. [Google Scholar] [CrossRef]
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Schubert, M.M.; Hackenberg, S.; van Veen, A.C.; Muhler, M.; Plzak, V.; Behm, R.J. CO Oxidation over Supported Gold Catalysts—“Inert” and “Active” Support Materials and Their Role for the Oxygen Supply during Reaction. J. Catal. 2001, 197, 113–122. [Google Scholar] [CrossRef]
- Bond, G.C.; Thompson, D.T. Catalysis by Gold. Catal. Rev. 1999, 41, 319–388. [Google Scholar] [CrossRef]
- Ilieva, L.; Petrova, P.; Pantaleo, G.; Zanella, R.; Sobczak, J.W.; Lisowski, W.; Kaszkur, Z.; Munteanu, G.; Yordanova, I.; Liotta, L.F.; et al. Alumina supported Au/Y-doped ceria catalysts for pure hydrogen production via PROX. Int. J. Hydrogen Energy 2018. [Google Scholar] [CrossRef]
- Moreno, M.S.; López, E.; Adrover, M.E.; Divins, N.J.; Llorca, J. CO-PrOx over nano-Au/TiO2: Monolithic catalyst performance and empirical kinetic model fitting. Int. J. Hydrogen Energy 2016, 41, 22043–22054. [Google Scholar] [CrossRef]
- Ruszel, M.; Grzybowska, B.; Łaniecki, M.; Wójtowski, M. Au/Ti-SBA-15 catalysts in CO and preferential (PROX) CO oxidation. Catal. Commun. 2007, 8, 1284–1286. [Google Scholar] [CrossRef]
- Storaro, L.; Lenarda, M.; Moretti, E.; Talon, A.; Porta, F.; Moltrasio, B.; Canton, P. Gold stabilized aqueous sols immobilized on mesoporous CeO2-Al2O3 as catalysts for the preferential oxidation of carbon monoxide. J. Colloid Interface Sci. 2010, 350, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Widmann, D.; Heenemann, M.; Diemant, T.; Biskupek, J.; Schlögl, R.; Behm, R.J. The role of electronic metal-support interactions and its temperature dependence: CO adsorption and CO oxidation on Au/TiO2 catalysts in the presence of TiO2 bulk defects. J. Catal. 2017, 354, 46–60. [Google Scholar] [CrossRef]
- Liu, X.; Wang, A.; Wang, X.; Mou, C.-Y.; Zhang, T. Au-Cu Alloy nanoparticles confined in SBA-15 as a highly efficient catalyst for CO oxidation. Chem. Commun. 2008, 3187–3189. [Google Scholar] [CrossRef] [PubMed]
- Ponrouch, A.; Garbarino, S.; Guay, D. Effect of the nanostructure on the CO poisoning rate of platinum. Electrochem. Commun. 2009, 11, 834–837. [Google Scholar] [CrossRef]
- Mohamed, Z.; Dasireddy, V.D.B.C.; Singh, S.; Friedrich, H.B. The preferential oxidation of CO in hydrogen rich streams over platinum doped nickel oxide catalysts. Appl. Catal. B Environ. 2016, 180, 687–697. [Google Scholar] [CrossRef]
- Sung, L.-Y.; Hwang, B.-J.; Hsueh, K.-L.; Tsau, F.-H. Effects of anode air bleeding on the performance of CO-poisoned proton-exchange membrane fuel cells. J. Power Sources 2010, 195, 1630–1639. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Zhang, Q.; Chmielewski, D.J.; Lauzze, K.C.; Inbody, M.A. Performance of CO preferential oxidation reactor with noble-metal catalyst coated on ceramic monolith for on-board fuel processing applications. Catal. Today 2005, 99, 271–283. [Google Scholar] [CrossRef]
- Xu, Y.; Mavrikakis, M. Adsorption and Dissociation of O2 on Gold Surfaces: Effect of Steps and Strain. J. Phys. Chem. B 2003, 107, 9298–9307. [Google Scholar] [CrossRef]
- Daté, M.; Okumura, M.; Tsubota, S.; Haruta, M. Vital Role of Moisture in the Catalytic Activity of Supported Gold Nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 2129–2132. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Wang, A.-Q.; Chi, Y.-S.; Lin, H.P.; Mou, C.-Y. Synergistic Effect in an Au-Ag Alloy Nanocatalyst: CO Oxidation. J. Phys. Chem. B 2005, 109, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.-Q.; Liu, J.-H.; Lin, S.D.; Lin, T.-S.; Mou, C.-Y. A novel efficient Au-Ag alloy catalyst system: Preparation, activity, and characterization. J. Catal. 2005, 233, 186–197. [Google Scholar] [CrossRef]
- Liao, X.; Chu, W.; Dai, X.; Pitchon, V. Bimetallic Au-Cu supported on ceria for PROX reaction: Effects of Cu/Au atomic ratios and thermal pretreatments. Appl. Catal. B Environ. 2013, 142–143, 25–37. [Google Scholar] [CrossRef]
- Phillips, J.; Weigle, J.; Herskowitz, M.; Kogan, S. Metal particle structure: Contrasting the influences of carbons and refractory oxides. Appl. Catal. A Gen. 1998, 173, 273–287. [Google Scholar] [CrossRef]
- Anderson, J.A.; Fernández-García, M.; Haller, G.L. Surface and Bulk Characterisation of Metallic Phases Present during CO Hydrogenation over Pd-Cu/KL Zeolite Catalysts. J. Catal. 1996, 164, 477–483. [Google Scholar] [CrossRef]
- Moretti, E.; Rodríguez-Aguado, E.; Molina, A.I.; Rodríguez-Castellón, E.; Talon, A.; Storaro, L. Sustainable photo-assisted CO oxidation in H2-rich stream by simulated solar light response of Au nanoparticles supported on TiO2. Catal. Today 2018, 304, 135–142. [Google Scholar] [CrossRef]
- Gómez-Cazalilla, M.; Mérida-Robles, J.M.; Gurbani, A.; Rodríguez-Castellón, E.; Jiménez-López, A. Characterization and acidic properties of Al-SBA-15 materials prepared by post-synthesis alumination of a low-cost ordered mesoporous silica. J. Solid State Chem. 2007, 180, 1130–1140. [Google Scholar] [CrossRef]
- Tu, C.-H.; Wang, A.-Q.; Zheng, M.-Y.; Wang, X.-D.; Zhang, T. Factors influencing the catalytic activity of SBA-15-supported copper nanoparticles in CO oxidation. Appl. Catal. A Gen. 2006, 297, 40–47. [Google Scholar] [CrossRef]
- Moretti, E.; Storaro, L.; Talon, A.; Lenarda, M. One-pot mesoporous Al–Ce–Cu oxide systems as catalysts for the preferential carbon monoxide oxidation (CO-PROX). Catal. Commun. 2009, 10, 522–527. [Google Scholar] [CrossRef]
- Colilla, M.; Martínez-Carmona, M.; Sánchez-Salcedo, S.; Ruiz-González, M.L.; González-Calbet, J.M.; Vallet-Regí, M. A novel zwitterionic bioceramic with dual antibacterial capability. J. Mater. Chem. B 2014, 2, 5639–5651. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhu, Y.; Xu, J.; Cheng, Z.; Li, H.; Li, X. Fluoroalcohol and fluorinated-phenol derivatives functionalized mesoporous SBA-15 hybrids: High-performance gas sensing toward nerve agent. J. Mater. Chem. 2012, 22, 2263–2270. [Google Scholar] [CrossRef]
- Qian, X.F.; Kamegawa, T.; Mori, K.; Li, H.X.; Yamashita, H. Calcium Phosphate Coatings Incorporated in Mesoporous TiO 2 /SBA-15 by a Facile Inner-pore Sol–gel Process toward Enhanced Adsorption-photocatalysis Performances. J. Phys. Chem. C 2013, 130916100033003. [Google Scholar] [CrossRef]
- Gao, X.; Bare, S.R.; Fierro, J.L.G.; Banares, M.A.; Wachs, I.E. Preparation and in-Situ Spectroscopic Characterization of Molecularly Dispersed Titanium Oxide on Silica. J. Phys. Chem. B 1998, 102, 5653–5666. [Google Scholar] [CrossRef]
- Górska, P.; Zaleska, A.; Kowalska, E.; Klimczuk, T.; Sobczak, J.W.; Skwarek, E.; Janusz, W.; Hupka, J. TiO2 photoactivity in vis and UV light: The influence of calcination temperature and surface properties. Appl. Catal. B Environ. 2008, 84, 440–447. [Google Scholar] [CrossRef]
- Moretti, E.; Molina, A.I.; Sponchia, G.; Talon, A.; Frattini, R.; Rodriguez-Castellon, E.; Storaro, L. Low-temperature carbon monoxide oxidation over zirconia-supported CuO–CeO2 catalysts: Effect of zirconia support properties. Appl. Surf. Sci. 2017, 403, 612–622. [Google Scholar] [CrossRef]
- Yang, J.; Liu, B.; Zhao, X. A visible-light-active Au-Cu(I)@Na2Ti6O13 nanostructured hybrid pasmonic photocatalytic membrane for acetaldehyde elimination. Chin. J. Catal. 2017, 38, 2048–2055. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, W.; Wang, T.; Wang, D.; Liu, L.; Ye, J. High performance Au-Cu alloy for enhanced visible-light water splitting driven by coinage metals. Chem. Commun. 2016, 52, 4694–4697. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, F.; Zhao, H.; Li, Y. Tailoring Cu valence and oxygen vacancy in Cu/TiO2 catalysts for enhanced CO2 photoreduction efficiency. Appl. Catal. B Environ. 2013, 134–135, 349–358. [Google Scholar] [CrossRef]
- Chang, F.-W.; Ou, T.-C.; Roselin, L.S.; Chen, W.-S.; Lai, S.-C.; Wu, H.-M. Production of hydrogen by partial oxidation of methanol over bimetallic Au–Cu/TiO2–Fe2O3 catalysts. J. Mol. Catal. A Chem. 2009, 313, 55–64. [Google Scholar] [CrossRef]
- Zhu, S.; Liang, S.; Gu, Q.; Xie, L.; Wang, J.; Ding, Z.; Liu, P. Effect of Au supported TiO2 with dominant exposed {0 0 1} facets on the visible-light photocatalytic activity. Appl. Catal. B Environ. 2012, 119–120, 146–155. [Google Scholar] [CrossRef]
- Zhang, L.; Blom, D.A.; Wang, H. Au-Cu2O Core-Shell Nanoparticles: A Hybrid Metal-Semiconductor Heteronanostructure with Geometrically Tunable Optical Properties. Chem. Mater. 2011, 23, 4587–4598. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Bansal, A.; Sekhon, J.S.; Verma, S.S. Scattering Efficiency and LSPR Tunability of Bimetallic Ag, Au, and Cu Nanoparticles. Plasmonics 2014, 9, 143–150. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sakamoto, H.; Sugano, Y.; Ichikawa, S.; Hirai, T. Pt–Cu Bimetallic Alloy Nanoparticles Supported on Anatase TiO2: Highly Active Catalysts for Aerobic Oxidation Driven by Visible Light. ACS Nano 2013, 7, 9287–9297. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Jurek, A. Progress, Challenge, and Perspective of Bimetallic TiO2-Based Photocatalysts. J. Nanomater. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Monga, A.; Bathla, A.; Pal, B. A Cu-Au bimetallic co-catalysis for the improved photocatalytic activity of TiO2 under visible light radiation. Sol. Energy 2017, 155, 1403–1410. [Google Scholar] [CrossRef]














| Sample | d100 (nm) | SBET (m2 g−1) | Vp (cm3 g−1) | dp (nm) |
|---|---|---|---|---|
| SBA | 8.74 | 654 | 0.50 | 5.0 |
| Ti-SBA | 8.57 | 393 | 0.32 | 4.8 |
| AuCu/SBA | 8.10 | 200 | 0.20 | 4.3 |
| AuCu/Ti-SBA | 8.25 | 237 | 0.19 | 4.3 |
| Au-Cu/Ti-SBA | 8.33 | 240 | 0.21 | 4.3 |
| SBA | APTES_SBA | Ti-SBA | APTES-Ti-SBA | |||||
|---|---|---|---|---|---|---|---|---|
| δ (ppm) | % | δ (ppm) | % | δ (ppm) | % | δ (ppm) | % | |
| Q4 | −110.1 | 69.4 | −111.1 | 63.1 | −110.2 | 80.1 | −110.2 | 58.6 |
| Q3 | −101.0 | 24.1 | −101.2 | 20.7 | −100.8 | 17.3 | −100.6 | 22.8 |
| Q2 | −91.9 | 6.5 | - | −91.8 | 2.6 | - | - | |
| T3 | - | - | −67.0 | 7.7 | - | - | −66.8 | 8.6 |
| T2 | - | - | −57.8 | 8.5 | - | - | −57.2 | 9.9 |
| Sample | Au/(Si + Ti) | Cu/(Si + Ti) | (Au + Cu)/(Si + Ti) | Au/Cu |
|---|---|---|---|---|
| AuCu/SBA | 0.018 | 0.029 | 0.047 | 0.65 |
| AuCu/Ti-SBA | 0.035 | 0.070 | 0.105 | 0.49 |
| Au-Cu/Ti-SBA | 0.071 | 0.053 | 0.123 | 1.33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso-Martín, I.; Infantes-Molina, A.; Talon, A.; Storaro, L.; Rodríguez-Aguado, E.; Rodríguez-Castellón, E.; Moretti, E. CO Preferential Photo-Oxidation in Excess of Hydrogen in Dark and Simulated Solar Light Irradiation over AuCu-Based Catalysts on SBA-15 Mesoporous Silica-Titania. Materials 2018, 11, 1203. https://doi.org/10.3390/ma11071203
Barroso-Martín I, Infantes-Molina A, Talon A, Storaro L, Rodríguez-Aguado E, Rodríguez-Castellón E, Moretti E. CO Preferential Photo-Oxidation in Excess of Hydrogen in Dark and Simulated Solar Light Irradiation over AuCu-Based Catalysts on SBA-15 Mesoporous Silica-Titania. Materials. 2018; 11(7):1203. https://doi.org/10.3390/ma11071203
Chicago/Turabian StyleBarroso-Martín, Isabel, Antonia Infantes-Molina, Aldo Talon, Loretta Storaro, Elena Rodríguez-Aguado, Enrique Rodríguez-Castellón, and Elisa Moretti. 2018. "CO Preferential Photo-Oxidation in Excess of Hydrogen in Dark and Simulated Solar Light Irradiation over AuCu-Based Catalysts on SBA-15 Mesoporous Silica-Titania" Materials 11, no. 7: 1203. https://doi.org/10.3390/ma11071203