5-Hydroxymethylfurfural (HMF) Production from Real Biomasses
Abstract
:1. Introduction
- Step 1:
- hydrolysis of glucan (a glucose-based polymer, e.g., cellulose and starch) to glucose catalyzed by a Brønsted acid;
- Step 2:
- isomerisation of glucose to fructose mediated by a Lewis acid;
- Step 3:
- dehydration of fructose to HMF facilitated by a Brønsted acid.
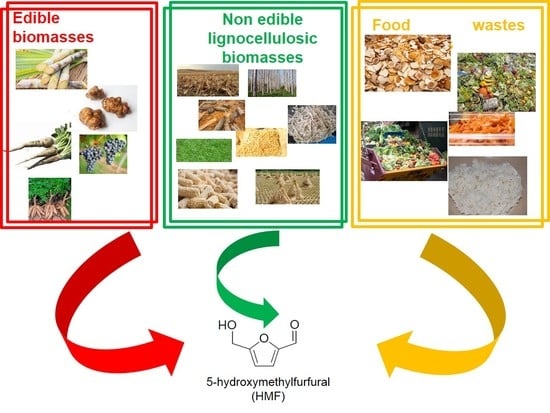
- (i)
- Edible biomasses;
- (ii)
- Non-edible lignocellulosic biomasses;
- (iii)
- Food wastes (FW).
2. HMF Production from Edible Biomasses
- (i)
- IL solvents (1-methyl-3-octylimidazolium chloride ([OMIM]Cl), 1-hexyl-3-methylimidazolium chloride ([HMIM]Cl), 1-ethyl-3-methylimidazolium chloride ([EMIM]Cl);
- (ii)
- reaction temperatures (80, 100 and 120 °C);
- (iii)
- chloride catalysts (CrCl2, CoCl2·6H2O, NiCl2·6H2O, ZnCl2, MgCl2);
- (iv)
- HCl concentrations (0, 0.1, 0.3, 0.5, and 1 M).
3. HMF Production from Non-Edible Lignocellulosic Biomasses
3.1. Liquid Ionic as a Solvent
3.2. Other Solvents
4. HMF from Food Waste
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Hulsey, M.J.; Yang, H.; Yan, N. Sustainable Routes for the Synthesis of Renewable Heteroatom-Containing Chemicals. ACS Sustain. Chem. Eng. 2018, 6, 5694–5707. [Google Scholar] [CrossRef]
- De Corato, U.; De Bari, I.; Viola, E.; Pugliese, M. Assessing the main opportunities of integrated biorefining from agrobioenergy co/by-products and agroindustrial residues into high-value added products associated to some emerging markets: A review. Renew. Sus. Energ. Rev. 2018, 88, 326–346. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Bicker, M.; Hirth, J.; Vogel, H. Dehydration of fructose to 5-hydroxymethylfurfural in sub- and supercritical acetone. Green Chem. 2003, 5, 280–284. [Google Scholar] [CrossRef]
- Artz, J.; Palkovits, R. Cellulose-based platform chemical: The path to application. Curr. Opin. Green Sustain. Chem. 2018, 14, 14–18. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Dumesic, J. Solvent Effects on Fructose Dehydration to 5-Hydroxymethylfurfural in Biphasic Systems Saturated with Inorganic Salts. Top. Catal. 2009, 52, 297–303. [Google Scholar] [CrossRef]
- Moreau, C.; Finiels, A.; Vanoye, L. Dehydration of fructose and sucrose into 5-hydroxymethylfurfural in the presence of 1-H-3-methyl imidazolium chloride acting both as solvent and catalyst. J. Mol. Catal. A Chem. 2006, 253, 165–169. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Roman-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Crisci, A.J.; Tucker, M.H.; Lee, M.Y.; Jang, S.G.; Dumesic, J.A.; Scott, S.L. Acid-Functionalized SBA-15-Type Silica Catalysts for Carbohydrate Dehydration. ACS Catal. 2011, 1, 719–728. [Google Scholar] [CrossRef]
- Chheda, J.N.; Roman-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Yong, G.; Zhang, Y.; Ying, J.Y. Efficient catalytic system for the selective production of 5-hydroxymethylfurfural from glucose and fructose. Angew. Chem. Int. Ed. 2008, 47, 9345–9348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hidajat, K.; Ray, A.K. Optimal design and operation of SMB bioreactor: Production of high fructose syrup by isomerization of glucose. Biochem. Eng. J. 2004, 21, 111–121. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Li, Y. Efficient and Selective Dehydration of Fructose to 5-Hydroxymethylfurfural Catalyzed by Brønsted-Acidic Ionic Liquids. ChemSusChem 2010, 3, 350–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Li, K.; Chen, L.; Liu, R.; Huang, X.; Ye, D. Conversion of fructose into 5-hydroxymethylfurfural catalyzed by recyclable sulfonic acid-functionalized metal–organic frameworks. Green Chem. 2014, 16, 2490–2499. [Google Scholar] [CrossRef]
- Lai, L.; Zhang, Y. The Effect of Imidazolium Ionic Liquid on the Dehydration of Fructose to 5-Hydroxymethylfurfural, and a Room Temperature Catalytic System. ChemSusChem 2010, 3, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, M.E.; Bogel-Lukasik, E.; Bogel-Lukasik, R. Ionic Liquid-Mediated Formation of 5-Hydroxymethylfurfural-A Promising Biomass-Derived Building Block. Chem. Rev. 2011, 111, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.B.; Raines, R.T. Simple Chemical Transformation of Lignocellulosic Biomass into Furans for Fuels and Chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.L.; Qi, W.R.; Su, X.; He, Z.M. Integrating enzymatic and acid catalysis to convert glucose into 5-hydroxymethylfurfural. Chem. Commun. 2010, 46, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Dumesic, J.A. Production and upgrading of 5-hydroxymethylfurfural using heterogeneous catalysts and biomass-derived solvents. Green Chem. 2013, 15, 85–90. [Google Scholar] [CrossRef]
- Zhao, S.; Cheng, M.; Li, J.; Tian, J.; Wang, X. One pot production of 5-hydroxymethylfurfural with high yield from cellulose by a Brønsted-Lewis-surfactant-combined heteropolyacid catalyst. Chem. Commun. 2011, 47, 2176–2178. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, Z.; Zhou, Y.; Song, J.; Fan, H.; Han, B. Direct conversion of inulin to 5-hydroxymethylfurfural in biorenewable ionic liquids. Green Chem. 2009, 11, 873–877. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Zhao, Z.K. Direct conversion of glucose and cellulose to 5-hydroxymethylfurfural in ionic liquid under microwave irradiation. Tetrahedron Lett. 2009, 50, 5403–5405. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L., Jr. Fast Transformation of Glucose and Di-/Polysaccharides into 5-Hydroxymethylfurfural by Microwave Heating in an Ionic Liquid/Catalyst System. ChemSusChem 2010, 3, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Morales, I.; Teckchandani-Ortiz, A.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Selective dehydration of glucose to 5-hydroxymethylfurfural on acidic mesoporous tantalum phosphate. Appl. Catal. B Environ. 2014, 144, 22–28. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, G.; Tang, X.; Wu, Z.; Xu, J.; Lin, L.; Liu, S. Catalytic conversion of carbohydrates into 5-hydroxymethylfurfural over cellulose-derived carbonaceous catalyst in ionic liquid. Bioresour. Technol. 2013, 148, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Fajgelj, A.; Belli, M.; Sansone, U. Biorefineries-Industrial Processes and Products, Status Quo and Future Directions; Kamm, B., Gruber, P., Kamm, M., Eds.; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Esteban, J.; Ladero, M. Food waste as a source of value-added chemicals and materials: A biorefinery perspective. Int. J. Food Sci. Technol. 2018, 53, 1095–1108. [Google Scholar] [CrossRef]
- Kläusli, T. AVA Biochem: Commercialising renewable platform chemical 5-HMF. Green Process Synth. 2014, 3, 235–236. [Google Scholar] [CrossRef]
- Haworth, W.N.; Wiggins, L.F. Improvements Relating to the Manufacture of 5-hydroxymethyl 2-furfural. U.K. Patent GB 600871, 1948. [Google Scholar]
- Garber, J.D.; Jones, R.E. Method for Producing 5-hydroxymethyl Furfural. U.S. Patent US3483228A, 9 December 1969. [Google Scholar]
- Rigal, L.; Gaset, A. Direct preparation of 5-hydroxymethyl-2-furancarboxaldehyde from polyholosides: A chemical valorisation of the Jerusalem artichoke (Helianthus tuberosus). Biomass 1983, 3, 151–163. [Google Scholar] [CrossRef]
- Seo, Y.; Han, J.I. Direct conversion from Jerusalem artichoke to hydroxymethylfurfural (HMF) using the Fenton reaction. Food Chem. 2014, 151, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Rapp, K.M. Process for Preparing Pure 5-Hydroxymethylfurfuraldehyde. U.S. Patent US4740605A, 26 April 1988. [Google Scholar]
- Yi, Y.B.; Lee, J.W.; Hong, S.S.; Choi, Y.H.; Chung, C.H. Acid mediated production of hydroxymethylfurfural from raw plant biomass with high inulin in an ionic liquid. J. Ind. Eng. Chem. 2011, 17, 6–9. [Google Scholar] [CrossRef]
- Yi, Y.B.; Lee, J.L.; Choi, Y.H.; Park, S.M.; Chung, C.H. Direct production of hydroxymethylfurfural from raw grape berry biomass using ionic liquids and metal chlorides. Environ. Chem. Lett. 2011, 10, 13–19. [Google Scholar] [CrossRef]
- Yi, Y.B.; Ha, M.G.; Lee, J.W.; Chung, C.H. New role of chromium fluoride: its catalytic action on the synthesis of hydroxymethylfurfural in ionic liquid using raw plant biomass and characterization of biomass hydrolysis. Chem. Eng. J. 2012, 180, 370–375. [Google Scholar] [CrossRef]
- Dutta, S.; De, S.; Alam, M.I.; Abu-Omar, M.M.; Saha, B. Direct conversion of cellulose and lignocellulosic biomass into chemicals and biofuel with metal chloride catalysts. J. Catal. 2012, 288, 8–15. [Google Scholar] [CrossRef]
- Kamm, B.; Gerhardt, M.; Dautzenberg, G. Catalytic processes of lignocellulosic feedstock conversion for production of furfural, levulinic acid, and formic acid-based fuel components. In New and Future Developments in Catalysis: Catalytic Biomass Conversion, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; p. 91. [Google Scholar]
- Alam, M.I.; De, S.; Dutta, S.; Saha, B. Solid-acid and ionic-liquid catalyzed one-pot transformation of biorenewable substrates into a platform chemical and a promising biofuel. RSC Adv. 2012, 2, 6890–6896. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Xia, Y.; Li, W.; Wang, H.; Zheng, C. Efficient process for the direct transformation of cellulose and carbohydrates to 5-(hydroxymenthyl)furfural with dual-core sulfonic acid ionic liquids and co-catalysts. RSC Adv. 2013, 3, 7782–7790. [Google Scholar] [CrossRef]
- Yan, L.; Liu, N.; Wang, Y.; Machida, H.; Qi, X. Production of 5-hydroxymethylfurfural from corn stalk catalyzed by corn stalk-derived carbonaceous solid acid catalyst. Bioresour. Technol. 2014, 173, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.V.; Lewis, D.; Chen, W.; Huang, H.; Alothman, Z.A.; Yamauchi, Y.; Wu, K.C. Combined treatments for producing 5-hydroxymethylfurfural (HMF) from lignocellulosic biomass. Catal. Today 2016, 278, 344–349. [Google Scholar] [CrossRef]
- Yuan, B.; Guan, J.; Peng, J.; Zhu, G.; Jiang, J. Green hydrolysis of corncob cellulose into 5-hydroxymethylfurfural using hydrophobic imidazole ionic liquids with a recyclable, magnetic metalloporphyrin catalyst. Chem. Eng. J. 2017, 330, 109–119. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Hou, Q.; Zhang, S.; Ju, M. Corn stalk conversion into 5-hydroxymethylfurfural by modified biochar catalysis in a multi-functional solvent. J. Clean. Prod. 2018, 187, 380–389. [Google Scholar] [CrossRef]
- Asghari, F.S.; Yoshida, H. Conversion of Japanese red pine wood (Pinus densiflora) into valuable chemicals under subcritical water conditions. Carbohydr. Res. 2010, 345, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Daengprasert, W.; Boonnoun, P.; Laosiripojana, N.; Goto, M.; Shotipruk, A. Application of sulfonated carbon-based catalyst for solvothermal conversion of Cassava waste to hydroxymethylfurfural and furfural. Ind. Eng. Chem. Res. 2011, 50, 7903–7910. [Google Scholar] [CrossRef]
- Käldström, M.; Kumar, N.; Tenho, M.; Mokeev, M.V.; Moskalenko, Y.E.; Murzin, D.Y. Catalytic Transformations of Birch Kraft Pulp. ACS Catal. 2012, 2, 1381–1393. [Google Scholar] [CrossRef]
- Iryania, D.A.; Kumagai, S.; Nonaka, M.; Sasakia, K.; Hirajima, T. Production of 5-hydroxymethyl Furfural from Sugarcane Bagasse under Hot Compressed Water. Procedia Earth Planet. Sci. 2013, 6, 441–447. [Google Scholar] [CrossRef]
- Zhang, T.; Kumara, R.; Wyman, C.E. Enhanced yields of furfural and other products by simultaneous solvent extraction during thermochemical treatment of cellulosic biomass. RSC Adv. 2013, 3, 9809–9819. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumara, R.; Wyman, C.E. THF co-solvent enhances hydrocarbon fuel precursor yields from lignocellulosic biomass. Green Chem. 2013, 15, 3140. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.; Abu-Omar, M.M. Conversion of carbohydrates and lignocellulosic biomass into 5-hydroxymethylfurfural using AlCl3·6H2O catalyst in a biphasic solvent system. Green Chem. 2012, 14, 509–513. [Google Scholar] [CrossRef]
- Lal, R. World crop residues production and implications of its use as a biofuel. Environ. Int. 2005, 31, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Yemis, O.; Mazza, G. Optimization of furfural and 5-hydroxymethylfurfural production from wheat straw by a microwave-assisted process. Bioresour. Technol. 2012, 109, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.M.; Nagane, N.; Kumar, R.; Wyman, C.E. Coupling metal halides with a co-solvent to produce furfural and 5-HMF at high yields directly from lignocellulosic biomass as an integrated biofuels strategy. Green Chem. 2014, 16, 3819–3829. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Rand, J.M.; Alonso, D.M.; Han, J.; Youngquist, J.T.; Maravelias, C.T.; Pfleger, B.F.; Dumesic, J.A. Nonenzymatic Sugar Production from Biomass Using Biomass-Derived γ-Valerolactone. Science 2014, 343, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, W.; Du, Z.; Wu, H.; Jameel, H.; Chang, H.; Ma, L. Conversion of corn stalk into furfural using a novel heterogeneous strong acid catalyst in γ-valerolactone. Bioresour. Technol. 2015, 198, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Mondal, J.; Bhaumik, A. Sulfonated Porous Polymeric Nanofibers as an Efficient Solid Acid Catalyst for the Production of 5-Hydroxymethylfurfural from Biomass. ChemCatChem 2015, 7, 3570–3578. [Google Scholar] [CrossRef]
- Seemala, B.; Haritos, V.; Tanksale, A. Levulinic Acid as a Catalyst for the Production of 5-Hydroxymethylfurfural and Furfural from Lignocellulose Biomass. ChemCatChem 2016, 8, 640–647. [Google Scholar] [CrossRef]
- Mirzaei, H.M.; Karimi, B. Sulphanilic acid as a recyclable bifunctional organocatalyst in the selective conversion of lignocellulosic biomass to 5-HMF. Green Chem. 2016, 18, 2282–2286. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, Z.; Xue, L.; Wang, X.; Jiang, Z. Highly efficient preparation of HMF from cellulose using temperature-responsive heteropolyacid catalysts in cascade reaction. Appl. Catal. B Environ. 2016, 196, 50–56. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, G.; Zhang, J.; Yu, H.; Wang, X. Efficient catalytic system for the direct transformation of lignocellulosic biomass to furfural and 5-hydroxymethylfurfural. Bioresour. Technol. 2017, 224, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Lu, Q.; Wang, X.; Wang, T.; Guo, H.; Cui, M.; Dong, C.; Yang, Y. Fast Pyrolysis of Corn Stalks at Different Growth Stages to Selectively Produce 4-Vinyl Phenol and 5-Hydroxymethyl Furfural. Waste Biomass Valor. 2018. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Suryadharma, M.S.; Pham, T.P.T.; Mahmood, R.; Balasubramanian, R. Heterogeneous catalyst-assisted thermochemical conversion of food waste biomass into 5-hydroxymethylfurfural. Bioresour. Technol. 2015, 178, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Chen, S.S.; Ok, Y.S.; Poon, C.S. Valorization of food waste into hydroxymethylfurfural: Dual role of metal ions in successive conversion steps. Bioresour. Technol. 2016, 219, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Khan, E.; Wang, L.; Ok, Y.S.; Poon, C.S. Valorization of cellulosic food waste into levulinic acid catalyzed by heterogeneous Bronsted acids: Temperature and solvent effects. Chem. Eng. J. 2017, 327, 328–335. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Chen, S.S.; Ok, Y.S.; Poon, C.S. Valorization of starchy, cellulosic, and sugary food waste into hydroxymethylfurfural by one-pot catalysis. Chemosphere 2017, 184, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.K.M.; Tsang, D.C.W.; Chen, S.S.; Wang, L.; Hunt, A.J.; Sherwood, J.; Vigier, K.D.O.; Jerome, F.; Ok, Y.S.; Poon, C.S. Polar aprotic solvent-water mixture as the medium for catalytic production of hydroxymethylfurfural (HMF) from bread waste. Bioresour. Technol. 2017, 245, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Chen, S.S.; Wang, L.; Ok, Y.S.; Poon, C.S. Catalytic valorization of starch-rich food waste into hydroxymethylfurfural (HMF): Controlling relative kinetics for high productivity. Bioresour. Technol. 2017, 237, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yu, I.K.M.; Chen, S.S.; Tsang, D.C.W.; Wang, L.; Xiong, X.; Zhang, S.; Ok, Y.S.; Kwon, E.E.; Song, H.; et al. Production of 5-hydroxymethylfurfural from starch-rich food waste catalyzed by sulfonated biochar. Bioresour. Technol. 2018, 252, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.K.M.; Ong, K.L.; Tsang, D.C.W.; Haque, M.A.; Kwan, T.H.; Chen, S.S.; Uisan, K.; Kulkarni, S.; Lin, C.S.K. Chemical transformation of food and beverage waste-derived fructose to hydroxymethylfurfural as a value-added product. Catal. Today 2018, 314, 70–77. [Google Scholar] [CrossRef]
- Isla, M.A.; Comelli, R.N.; Seluy, L.G. Wastewaster from the soft drinks industry source for bioethanol production. Bioresour. Technol. 2013, 136, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef] [PubMed]









| Biomass | Liquid Ionic | Homogeneous Catalyst | Heterogeneous Catalyst | Year | Ref | HMF Yield |
|---|---|---|---|---|---|---|
| Cane juice | x | 1948 | [31] | 11% | ||
| Cane juice | x | x | 1969 | [32] | 44% | |
| Jerusalem artichoke | x | 1983 | [33] | 57% | ||
| Jerusalem artichoke | x | 2014 | [34] | 35% | ||
| Chicory roots | x | 1988 | [35] | 9% | ||
| Chicory roots | x | x | 2011 | [36] | 51% | |
| Grape berries | x | x | 2011 | [37] | 10% | |
| Tapioca roots | x | x | 2012 | [38] | 53% |
| Biomass | LIs | Homogeneous Catalyst | Heterogeneous Catalyst | Other | Year | Ref | HMF Yield |
|---|---|---|---|---|---|---|---|
| Corn stover | x | x | 2009 | [19] | 48% | ||
| Pine sawdust | x | x | 2009 | [19] | 19% | ||
| Sugarcane bagasse | x | x | x | mw | 2012 | [39] | 42% |
| Foxtail weed | x | x | mw | 2012 | [41] | 58% | |
| Filter paper | x | x | 2013 | [42] | 40% | ||
| Corn stalks | x | x | 2014 | [43] | 44% | ||
| Wood chop rise straw | x | x | 2016 | [44] | 79 mol% | ||
| Corncob | x | x | 2017 | [45] | 66% | ||
| Corn stalk | x | x | 2018 | [46] | 63% | ||
| Red pine wood | x | scwa | 2010 | [47] | 25% | ||
| Cassava wastes | x | 2011 | [48] | 12% | |||
| Birch kraft pulp | x | 2012 | [49] | 8% | |||
| Corn stover | x | mw | 2012 | [50] | 19% | ||
| Pine wood | x | 2012 | [53] | 35% | |||
| Grass | x | 2012 | [53] | 23% | |||
| Poplar | x | 2012 | [53] | 26% | |||
| Wheat straw | x | mw | 2012 | [55] | 3.4% | ||
| Sugarcane bagasse | hcw | 2013 | [50] | 3% | |||
| Maple wood | x | 2013 | [51] | 47% | |||
| Maple wood | x | 2013 | [52] | 21% | |||
| Maple wood | x | 2014 | [56] | 51% | |||
| Corn stover | x | 2014 | [56] | 45% | |||
| Corn stover | x | 2014 | [57] | 60% | |||
| Corn stover | x | 2015 | [58] | 19.5% | |||
| Sugarcane bagasse | x | mw | 2015 | [59] | 20% | ||
| Pinewood sawdust | x | 2016 | [60] | <10% | |||
| Straw and barley husk | x | 2016 | [61] | 41% | |||
| Corn stover | x | 2016 | [62] | 27% | |||
| Corncob | x | 2017 | [63] | 32% | |||
| Corn stalks | py | 2018 | [64] | 5% |
| Biomass | Liquid Ionic | Homogeneous Catalyst | Heterogeneous Catalyst | Year | Ref | HMF Yield |
|---|---|---|---|---|---|---|
| Food waste | x | 2015 | [65] | 4.7% | ||
| Food waste | x | 2016 | [66] | 9.5% | ||
| Food waste | x | 2017 | [67] | 16% | ||
| Cooked rise or penne waste | x | 2017 | [68] | 23% | ||
| Fruits waste | x | 2017 | [68] | 13% | ||
| Bread waste | x | 2017 | [69] | 27 mol% | ||
| Bread waste | x | 2017 | [70] | 30 mol% | ||
| Bread waste | x | 2017 | [71] | 30 mol% | ||
| Beverage + Food wastes | x | 2018 | [72] | 71 mol% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses. Molecules 2018, 23, 2201. https://doi.org/10.3390/molecules23092201
Menegazzo F, Ghedini E, Signoretto M. 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses. Molecules. 2018; 23(9):2201. https://doi.org/10.3390/molecules23092201
Chicago/Turabian StyleMenegazzo, Federica, Elena Ghedini, and Michela Signoretto. 2018. "5-Hydroxymethylfurfural (HMF) Production from Real Biomasses" Molecules 23, no. 9: 2201. https://doi.org/10.3390/molecules23092201
APA StyleMenegazzo, F., Ghedini, E., & Signoretto, M. (2018). 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses. Molecules, 23(9), 2201. https://doi.org/10.3390/molecules23092201