Efficient Chemo-Enzymatic Transformation of Animal Biomass Waste for Eco-Friendly Leather Production
Abstract
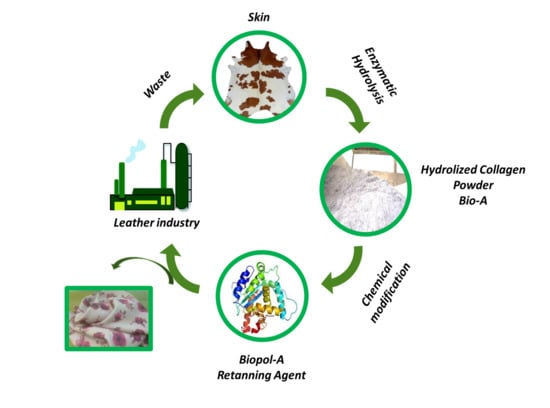
:1. Introduction
2. Results and Discussion
2.1. Enzymatic Hydrolysis
2.2. Characterization of Bio-A, Bio-IA and Bio-Ac
2.2.1. Elemental Analysis
2.2.2. NMR Studies
2.2.3. FTIR Analysis
2.2.4. GPC Studies
2.3. Characterization of Bio-Ac Retanned Leather
2.3.1. Application Tests: Leather Retanning
2.3.2. SEM Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Enzymatic Hydrolysis
3.4. Structural Characterization
3.5. Retanning Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Conseil National du Cuir. Available online: https://conseilnationalducuir.org/en/press/releases/2018-01-24 (accessed on 13 June 2019).
- Black, M.; Canova, M.; Rydin, S.; Scalet, B.M.; Roudier, S.; Sancho, D.L. Best Available Techniques (BAT) Reference Document for the Tanning of Hides and Skins. Industrial Emissions Directive 2010/75/EU; Publications Office of the European Union: Luxemburg, 2013. [Google Scholar]
- Ilou, I.; Souabi, S.; Digua, K. Quantification of Pollution Discharges from Tannery Wastewater and Pollution Reduction by Pre-Treatment Station. Int. J. Sci. Res. 2014, 3, 1706–1715. [Google Scholar]
- Beghetto, V.; Lodovico, A.; Gatto, V.; Samiolo, R.; Scrivanti, A. Sustainable use of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride as metal free tanning agent. J. Clean. Prod. 2019, 220, 864–872. [Google Scholar] [CrossRef]
- European Commission. Being Wise with Waste: The EU’s Approach to Waste Management; Publication Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Closing the Loop—An EU Action Plan for the Circular Economy; European Environment Agency (EEA): Copenhagen, Denmark, 2015; COM(2015)614 final.
- Pfaltzgraff, L.A.; De Bruyn, M.; Cooper, E.C.; Budarin, V.; Clark, J.H. Food waste biomass: A resource for high-value chemicals. Green Chem. 2013, 15, 307–314. [Google Scholar] [CrossRef]
- Catalina, M.; Cot, J.; Balu, A.M.; Serrano-Ruiz, J.C.; Luque, R. Tailor-made biopolymers from leather waste valorisation. Green Chem. 2012, 14, 308–312. [Google Scholar] [CrossRef]
- Sundar, V.J.; Gnanamani, A.; Muralidharan, C.; Chandrababu, N.K. Recovery and utilization of proteinous wastes of leather making: A review. Rev. Environ. Sci. Biotechnol. 2011, 10, 151–163. [Google Scholar] [CrossRef]
- Sundar, V.J.; Raghavarao, J.; Muralidharan, C.; Mandal, A.B. Recovery and Utilization of Chromium-Tanned Proteinous Wastes of Leather Making: A Review. Crit. Rev. Environ. Sci. Technol. 2011, 41, 2048–2075. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Taylor, M.M.; DiMaio, G.L.; Brown, E.M.; Marmer, G.L.; Carriò, R.; Celma, P.J.; Cot, J. Processing of leather waste: Pilot scale studies on chrome shavings. Isolation of potentially valuable protein products and chromium. Waste Manag. 1998, 18, 211–218. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, J.; Han, W. The status and developments of leather solid waste treatment: A mini-review. Waste Manag. Res. 2016, 34, 399–408. [Google Scholar] [CrossRef]
- Dang, X.; Yang, M.; Zhang, B.; Chen, H.; Wang, Y.; Mandal, A.B. Recovery and utilization of collagen protein powder extracted from chromium leather scrap waste. Environ. Sci. Pollut. Res. 2019, 7, 7277–7283. [Google Scholar] [CrossRef]
- Pahlawan, I.F.; Sutyasmi, S.; Griyanitasari, G. Hydrolysis of leather shavings waste for protein binder. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Malang, Indonesia, 18–20 September 2018; IOP Publishing: Bristol, UK, 2019; Volume 230, pp. 1–6. [Google Scholar]
- Narasimhan, K.; Sehgal, P.K.; Joseph, K.T. Condensation of aromatic sulfonic acid with hydrolysates from leather wastes. J. Am. Leather Chem. Assoc. 1980, 75, 211–215. [Google Scholar]
- Zehra, B.; Nawaz, H.R.; Solangi, B.A.; Nadeem, U.; Zeeshan, M. Preparation of double action surfactant using protein hydrolyzate from fleshing waste and its utilization as a lubricant with retanning property in leather making. Proc. Pak. Acad. Sci. 2014, 51, 337–343. [Google Scholar]
- Santos, L.M.; Gutterres, M. Reusing of a hide waste for leather fatliquoring. J. Clean. Prod. 2007, 15, 12–16. [Google Scholar] [CrossRef]
- Munoz, J.M.; Maldonado, M.V.; Rangel, A.S. Development of a tanning process based on using hydrolyzated material collected from leather scrap. J. Am. Leather Chem. Assoc. 2002, 97, 83–87. [Google Scholar]
- Yılmaz, O.; Kantarli, I.C.; Yuksel, M.; Saglam, M.; Yanik, J. Conversion of leather wastes to useful products. Resour. Conserv. Recycl. 2007, 49, 436–448. [Google Scholar] [CrossRef]
- Afsar, A.; Aslan, A.; Gulumser, G.; Ocak, B. A study on usability of collagen hydrolysate along with oxazolidine in leather processing. Text. App. 2010, 20, 37–40. [Google Scholar]
- Zehra, B.; Nawaz, H.R.; Solangi, B.A.; Nadeem, U. Extraction of protein from chrome shavings, modification with acrylic monomers and further re-utilization in leather processing. Am. Sci. Res. J. Eng. Technol. Sci. 2019, 1, 98–104. [Google Scholar]
- Gunther, R.C. Chemistry and Characteristics of Enzyme-Modified Whipping Proteins. J. Am. Oil Chem. Soc. 1979, 56, 345–349. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Velappan, K.C.; Chandra Babu, N.K.; Sadulla, S. Solid wastes generation in the leather industry and its utilization for cleaner environment—A Review. J. Sci. Ind. Res. 2006, 65, 541–548. [Google Scholar]
- Taylor, M.M.; Dieffendorf, E.J.; Na, G.C. Enzymic treatment of chrome shaving. Leather Manuf. 1990, 85, 264–274. [Google Scholar]
- Cavani, L.; Margon, A.; Sciubba, L.; Ciavatta, C.; Marzadori, C. What we talk about when we talk about protein hydrolizate-based biostimulants. AIMS Agric. Food 2017, 2, 221–232. [Google Scholar] [CrossRef]
- Convington, A.D. Tanning Chemistry: The Science of Leather; Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar]
- Vatansever, B.; Binici, B. A quantitative method for the measurement of hydrolysed type-I collagen protein in dietary supplement syrup using HPLC-SEC-UV technique. J. Chem. Metrol. 2015, 9, 1–15. [Google Scholar]
- Penkova, R.; Goshev, I.; Gorinstein, S.; Nedkov, P. Stabilizing effect of glycerol on collagen type I isolated from different species. Food Chem. 1999, 66, 483–487. [Google Scholar] [CrossRef]
- Valerio, O.; Pin, J.M.; Misra, M.; Mohanty, A.K. Synthesis of Glycerol-Based Biopolyesters as Toughness Enhancers for Polylactic Acid Bioplastic through Reactive Extrusion. ACS Omega 2016, 1, 1284–1295. [Google Scholar] [CrossRef]
- Ristić, I.; Miletić, A.; Cakić, S.; Govedarica, O.; Janković, M.; Sinadinović-Fišer, S.; Budinski-Simendić, J. The synthesis of polyacrylic acid with controlled molecular weight. Phys. Chem. 2016, 2, 685–688. [Google Scholar]
- Molnar, R.M.; Bodnar, M.; Hartmann, J.F.; Borbély, J. Preparation and characterization of poly(acrylic acid)-based nanoparticles. Colloid Polym. Sci. 2009, 287, 739–744. [Google Scholar] [CrossRef]
- Doyle, B.B. Infrared Spectroscopy of Collagen and Collagen-Like Polypeptides. Biopolymers 1975, 14, 937–957. [Google Scholar] [CrossRef]
- Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Camacho, N.P.; West, P.; Torzilli, P.A.; Mendelsohn, R. FTIR Microscopic Imaging of Collagen and Proteoglycan in Bovine Cartilage. Biopolymers 2001, 62, 1–8. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Lisperguer, J.; Nunez, C.; Perez-Guerrero, P. Structure and thermal properties of maleated lignin-recycled polystyrene composites. J. Chil. Chem. Soc. 2013, 58, 1937–1940. [Google Scholar] [CrossRef]
- Salehpour, S.; Dubé, M.A. Reaction Monitoring of Glycerol Step-Growth Polymerization Using ATR-FTIR Spectroscopy. Macromol. React. Eng. 2012, 6, 85–92. [Google Scholar] [CrossRef]
- Castiello, D.; Chiellini, E.; Cinelli, P.; D’antone, S.; Puccini, M.; Salvadori, M.; Seggiani, M. Polyethylene-Collagen hydrolizate thermoplastic blends: Thermal and mechanical properties. J. Appl. Polym. Sci. 2009, 114, 3827–3834. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L. Recent research progress on leather fatliquoring agents. Polym. Plast. Technol. 2009, 48, 285–291. [Google Scholar] [CrossRef]
- Liquiang, J.; Yanwei, W.; Yulu, W.; Yanchun, L. Preparation and application of an amphiphilic acrylic copolymer as a retanning agent. Sltc J. 2014, 98, 222–228. [Google Scholar]
- Bianli, R.; Qi, F.; Yuye, C.; Yunjun, L.; Xianglong, Z. The relantionship between the organoleptic properties of leather and the aggregate structure of collagen fibres. J. Soc. Leather Technol. Chem. 2018, 2012, 289–292. [Google Scholar]
- UL LLC. Vehicle Interior Air Quality: Addressing Chemical Exposure in Automobiles; UL LLC: Northbrook, IL, USA, 2015. [Google Scholar]
- Krishnamoorthy, G.; Sadulla, S.; Sehgal, P.K.; Manda, A.B. Greener approach to leather tanning process: D-Lysine aldehyde as novel tanning agent for chrome-free tanning. J. Clean. Prod. 2013, 42, 277–286. [Google Scholar] [CrossRef]
- Nashy, E.H.A.; Hussein, A.I.; Essa, M.M. Retanning agents for chrome tanned leather based on emulsion nano-particles of styrene/butyl acrylate copolymers. N. Y. Sci. J. 2010, 3, 13–21. [Google Scholar]
- Sivasubramanian, S.; Manohar, B.M.; Rajaram, A.; Puvanakrishnan, R. Ecofriendly lime and sulfide free enzymatic dehairing of skins and hides using a bacterial alkaline protease. Chemosphere 2008, 70, 1015–1024. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, J.; Wang, Y.; Shi, B. Modification of leather split by in situ polymerization of acrylates. Int. J. Polym. Sci. 2016, 2016, 1–7. [Google Scholar] [CrossRef]







| Analysed Parameter | Bio-A |
|---|---|
| Organic nitrogen (N) % | 15.4 ± 0.3 |
| Total carbon (C) % | 42.6 ± 1.0 |
| Dry matter % | 94.2 ± 2.0 |
| Ashes % | 6.6 ± 0.5 |
| pH | 5.7 ± 0.5 |
| Total aminoacids % | 96.8 ± 3.0 |
| Free aminoacids % | 1.8 ± 0.4 |
| Hydrolysis degree | 9.2 ± 1.1 |
| Cr (III) (mg/Kg) | 40 ± 2 |
| Cr (VI) (mg/Kg) | <0.5 (a) |
| Salmonella spp | ABSENT |
| Coliforms (UFC/g) | <10 |
| Aminoacids (a) | Total (%) | Free (%) (b) | Aminoacids (a) | Total (%) | Free (%) (b) |
|---|---|---|---|---|---|
| Hydroxyproline | 11.0 | <lq | Cysteine | <lq | <lq |
| Aspartic acid | 4.9 | <lq | Tyrosine | 1.0 | <lq |
| Serine + asparagine | 3.4 | 0.1 | Hydroxylisine | 1.2 | <lq |
| Glutamic acid | 9.2 | 0.2 | Valine | 2.3 | <lq |
| Glycine | 22.2 | 0.2 | Metionine | 0.9 | <lq |
| Hystidine + glutamine | 0.9 | <lq | Ornitine | 0.5 | <lq |
| Arginine | 8.2 | 0.1 | Lysine | 3.4 | <lq |
| Threonine | 1.1 | 1.0 | Isoleucine | 1.4 | <lq |
| Alanine | 7.9 | 0.1 | Leucine | 2.9 | <lq |
| Proline | 12.5 | 0.1 | Phenilalanine | 1.9 | <lq |
| γ - Aminobutyric acid | <lq | <lq | Tryptophan | <lq | <lq |
| α - Aminobutyric acid | <lq | <lq | Total | 96.8 | 1.8 |
| Sample | Mw (Da) |
|---|---|
| Bio-A | 5149 |
| Bio-IA | 7722 |
| Bio-Ac | 42,400 |
| Recipe a | Retanning | Light Fastness b | Fogging Refractometric c | Fogging Gravimetric d |
|---|---|---|---|---|
| Acrylic Biopolymer | Bio-Ac (6%) | 5 | 99 | 0.8 |
| Standard | Acrylic resin (6%) | 4 | 96 | 2.3 |
| Standard | Phenolic Syntan (6%) | 4 | 94 | 3.5 |
| Recipe a | Retanning | Grain Distension b | Grain Strength b | Tear Strength c | ||
|---|---|---|---|---|---|---|
| Elongation (nm) | Load (Kg) | Elongation (nm) | Load (Kg) | |||
| Acrylic Biopolymer | Bio-Ac (6%) | 8.36 | 18 | 12.55 | 40 | 0.8 |
| Standard | Acrylic resin (6%) | 8.73 | 19 | 11.65 | 30 | 2.3 |
| Standard | Phenolic Syntan (6%) | 6.39 | 16 | 10.67 | 48 | 3.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sole, R.; Taddei, L.; Franceschi, C.; Beghetto, V. Efficient Chemo-Enzymatic Transformation of Animal Biomass Waste for Eco-Friendly Leather Production. Molecules 2019, 24, 2979. https://doi.org/10.3390/molecules24162979
Sole R, Taddei L, Franceschi C, Beghetto V. Efficient Chemo-Enzymatic Transformation of Animal Biomass Waste for Eco-Friendly Leather Production. Molecules. 2019; 24(16):2979. https://doi.org/10.3390/molecules24162979
Chicago/Turabian StyleSole, Roberto, Lorenzo Taddei, Clizia Franceschi, and Valentina Beghetto. 2019. "Efficient Chemo-Enzymatic Transformation of Animal Biomass Waste for Eco-Friendly Leather Production" Molecules 24, no. 16: 2979. https://doi.org/10.3390/molecules24162979