Glycerol Valorization towards a Benzoxazine Derivative through a Milling and Microwave Sequential Strategy
Abstract
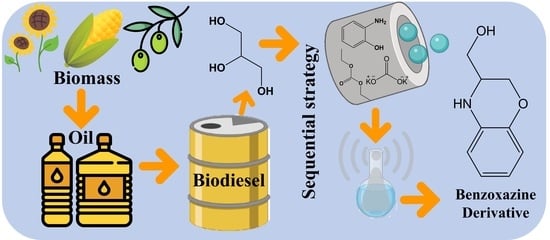
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Mechanochemically-Assisted Approach
3.2. Microwave-Assisted Approach
3.3. Combined Microwave and Mechanochemical-Assisted Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Balu, A.M.; Muñoz-Batista, M.J.; Luque, R. Environmental Catalysis: Present and Future. Chem. Cat. Chem. 2019, 11, 18–38. [Google Scholar] [CrossRef]
- Tokarska, K.B.; Stolpe, M.B.; Sippel, S.; Fischer, E.M.; Smith, C.J.; Lehner, F.; Knutti, R. Past warming trend constrains future warming in CMIP6 models. Sci. Adv. 2020, 6, eaaz9549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Lyu, Y.; Tian, J.; Zhao, J.; Ye, N.; Zhang, Y.; Chen, L. Review of waste biorefinery development towards a circular economy: From the perspective of a life cycle assessment. Renew. Sustain. Energy Rev. 2021, 139, 110716. [Google Scholar] [CrossRef]
- Liao, Y.; Koelewijn, S.-F.; Bossche, G.V.d.; Aelst, J.V.; Bosch, S.V.d.; Renders, T.; Navare, K.; Nicolaï, T.; Aelst, K.V.; Maesen, M.; et al. A sustainable wood biorefinery for low-carbon footprint chemicals production. Science 2020, 367, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Paone, E.; Tabanelli, T.; Mauriello, F. The rise of lignin biorefinery. Curr. Opin. Green Sustain. Chem. 2020, 24, 1–6. [Google Scholar] [CrossRef]
- Benessere, V.; Cucciolito, M.E.; De Santis, A.; Di Serio, M.; Esposito, R.; Melchiorre, M.; Nugnes, F.; Paduano, L.; Ruffo, F. A Sustainable Process for the Production of Varnishes Based on Pelargonic Acid Esters. J. Am. Oil Chem. Soc. 2019, 96, 443–451. [Google Scholar] [CrossRef]
- Melchiorre, M.; Amendola, R.; Benessere, V.; Cucciolito, M.E.; Ruffo, F.; Esposito, R. Solvent-free transesterification of methyl levulinate and esterification of levulinic acid catalyzed by a homogeneous iron(III) dimer complex. Mol. Catal. 2020, 483, 110777. [Google Scholar] [CrossRef]
- Melchiorre, M.; Benessere, V.; Cucciolito, M.E.; Melchiorre, C.; Ruffo, F.; Esposito, R. Direct and Solvent-Free Oxidative Cleavage of Double Bonds in High-Oleic Vegetable Oils. Chem. Select 2020, 5, 1396–1400. [Google Scholar] [CrossRef]
- Khodadadi, M.R.; Malpartida, I.; Tsang, C.-W.; Lin, C.S.K.; Len, C. Recent advances on the catalytic conversion of waste cooking oil. Mol. Catal. 2020, 494, 111128. [Google Scholar] [CrossRef]
- Essamlali, Y.; Amadine, O.; Larzek, M.; Len, C.; Zahouily, M. Sodium modified hydroxyapatite: Highly efficient and stable solid-base catalyst for biodiesel production. Energy Convers. Manag. 2017, 149, 355–367. [Google Scholar] [CrossRef]
- Varma, R.S.; Len, C. Glycerol valorization under continuous flow conditions-recent advances. Curr. Opin. Green Sustain. Chem. 2019, 15, 83–90. [Google Scholar] [CrossRef]
- Corrêa, I.; Faria, R.P.V.; Rodrigues, A.E. Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives. Sustain. Chem. 2021, 2, 286–324. [Google Scholar] [CrossRef]
- Aroua, M.K.; Cognet, P. Editorial: From Glycerol to Value-Added Products. Front. Chem. 2020, 8, 69. [Google Scholar] [CrossRef]
- Akinnawo, C.A.; Mosia, L.; Alimi, O.A.; Oseghale, C.O.; Fapojuwo, D.P.; Bingwa, N.; Meijboom, R. Eco-friendly synthesis of valuable fuel bio-additives from glycerol. Catal. Commun. 2021, 152, 106287. [Google Scholar] [CrossRef]
- Kostyniuk, A.; Bajec, D.; Djinović, P.; Likozar, B. One-step synthesis of glycidol from glycerol in a gas-phase packed-bed continuous flow reactor over HZSM-5 zeolite catalysts modified by CsNO3. Chem. Eng. J. 2020, 394, 124945. [Google Scholar] [CrossRef]
- Arora, S.; Gosu, V.; Kumar, U.K.A.; Subbaramaiah, V. Valorization of glycerol into glycerol carbonate using the stable heterogeneous catalyst of Li/MCM-41. J. Clean. Prod. 2021, 295, 126437. [Google Scholar] [CrossRef]
- Bartoli, M.; Zhu, C.; Chae, M.; Bressler, D.C. Glycerol Acetylation Mediated by Thermally Hydrolysed Biosolids-Based Material. Catalysts 2020, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renew. Sustain. Energy Rev. 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- Truscello, A.M.; Gambarotti, C.; Lauria, M.; Auricchio, S.; Leonardi, G.; Shisodia, S.U.; Citterio, A. One-pot synthesis of aryloxypropanediols from glycerol: Towards valuable chemicals from renewable sources. Green Chem. 2013, 15, 625–628. [Google Scholar] [CrossRef]
- Garg, V.; Kumar, A.; Chaudhary, A.; Agrawal, S.; Tomar, P.; Sreenivasan, K.K. Synthesis, biological evaluation and molecular docking studies of 1,3-benzoxazine derivatives as potential anticancer agents. Med. Chem. Res. 2013, 22, 5256–5266. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Ong, M.Y. A Review of Microwave-Assisted Reactions for Biodiesel Production. Bioengineering 2017, 4, 57. [Google Scholar] [CrossRef] [Green Version]
- Kokel, A.; Schäfer, C.; Török, B. Application of microwave-assisted heterogeneous catalysis in sustainable synthesis design. Green Chem. 2017, 19, 3729–3751. [Google Scholar] [CrossRef]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. Int. Ed. 2020, 59, 1018–1029. [Google Scholar] [CrossRef]
- Andersen, J.; Mack, J. Mechanochemistry and organic synthesis: From mystical to practical. Green Chem. 2018, 20, 1435–1443. [Google Scholar] [CrossRef]
- Tan, D.; García, F. Main group mechanochemistry: From curiosity to established protocols. Chem. Soc. Rev. 2019, 48, 2274–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Batista, M.J.; Rodriguez-Padron, D.; Puente-Santiago, A.R.; Luque, R. Mechanochemistry: Toward Sustainable Design of Advanced Nanomaterials for Electrochemical Energy Storage and Catalytic Applications. ACS Sustain. Chem. Eng. 2018, 6, 9530–9544. [Google Scholar] [CrossRef]
- Seo, T.; Ishiyama, T.; Kubota, K.; Ito, H. Solid-state Suzuki–Miyaura cross-coupling reactions: Olefin-accelerated C–C coupling using mechanochemistry. Chem. Sci. 2019, 10, 8202–8210. [Google Scholar] [CrossRef] [Green Version]
- Yeboue, Y.; Jean, M.; Subra, G.; Martinez, J.; Lamaty, F.; Métro, T.-X. Epimerization-Free C-Term Activation of Peptide Fragments by Ball Milling. Org. Lett. 2021, 23, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, J.D.; O’Reilly, D.; Friščić, T.; Damha, M.J. Mechanochemical Synthesis of Short DNA Fragments. Chem. Eur. J. 2020, 26, 8857–8861. [Google Scholar] [CrossRef] [PubMed]
- Espro, C.; Rodríguez-Padrón, D. Re-thinking organic synthesis: Mechanochemistry as a greener approach. Curr. Opin. Green Sustain. Chem. 2021, 30, 100478. [Google Scholar] [CrossRef]
- Bernasconi, C.F.; Howard, K.A.; Kanavarioti, A. Nucleophilic addition to olefins. 11. Kinetics of the reversible hydrolysis of benzylidenemalononitrile in water. J. Am. Chem. Soc. 1984, 106, 6827–6835. [Google Scholar] [CrossRef]
- Franco, A.; De, S.; Balu, A.M.; Romero, A.A.; Luque, R. Integrated Mechanochemical/Microwave-Assisted Approach for the Synthesis of Biogenic Silica-Based Catalysts from Rice Husk Waste. ACS Sustain. Chem. Eng. 2018, 6, 11555–11562. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. ChemInform Abstract: Environmentally Benign Chemical Synthesis via Mechanochemical Mixing and Microwave Irradiation; RSC Publishing: Cambridge, UK, 2010; Volume 41. [Google Scholar] [CrossRef]
- Guérin, B.; Fernandes, D.M.; Daran, J.-C.; Agustin, D.; Poli, R. Investigation of induction times, activity, selectivity, interface and mass transport in solvent-free epoxidation by H2O2 and TBHP: A study with organic salts of the [PMo12O40]3− anion. New J. Chem. 2013, 37, 3466–3475. [Google Scholar] [CrossRef]
- Gervais, T.; Jensen, K.F. Mass transport and surface reactions in microfluidic systems. Chem. Eng. Sci. 2006, 61, 1102–1121. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L.; Tumanov, I.A.; Boldyreva, E.V. The effect of ball mass on the mechanochemical transformation of a single-component organic system: Anhydrous caffeine. J. Mater. Sci 2018, 53, 13380–13389. [Google Scholar] [CrossRef] [Green Version]
- Howard, J.L.; Cao, Q.; Browne, D.L. Mechanochemistry as an emerging tool for molecular synthesis: What can it offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef] [Green Version]
- Baig, R.B.N.; Varma, R.S. Alternative energy input: Mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Alegria, E.C.B.A.; Kopylovich, M.N.; Ferraria, A.M.; Botelho do Rego, A.M.; Pombeiro, A.J.L. Comparison of microwave and mechanochemical energy inputs in the catalytic oxidation of cyclohexane. Dalton Trans. 2018, 47, 8193–8198. [Google Scholar] [CrossRef]



| Entry | Mechanochemical Conditions | Microwave Conditions/Conventional Heating | C * (%) | S ** (P2, %) | Y *** (%) |
|---|---|---|---|---|---|
| 1 | 60 min, 350 rpm | - | <1 | <1 | <1 |
| 2 | 120 min, 350 rpm | - | <1 | <1 | <1 |
| 3 | 110 °C, 300 W, 30 min | 10 | 32 | 3 | |
| 4 | - | 110 °C, 300 W, 60 min | 9 | 92 | 8 |
| 5 | - | 110 °C, 300 W, 120 min | 12 | 45 | 5 |
| 6 | - | 150 °C, 300 W, 60 min | 25 | 35 | 9 |
| 7 b | 60 min, 350 rpm | 110 °C, 60 min | <5 | <1 | <1 |
| 8 a | 60 min, 350 rpm | 110 °C, 300 W, 60 min | 38 | 93 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Pastor, M.Á.; Espro, C.; Selva, M.; Perosa, A.; Romero Reyes, A.A.; Osman, S.M.; Luque, R.; Rodríguez-Padrón, D. Glycerol Valorization towards a Benzoxazine Derivative through a Milling and Microwave Sequential Strategy. Molecules 2022, 27, 632. https://doi.org/10.3390/molecules27030632
Torres-Pastor MÁ, Espro C, Selva M, Perosa A, Romero Reyes AA, Osman SM, Luque R, Rodríguez-Padrón D. Glycerol Valorization towards a Benzoxazine Derivative through a Milling and Microwave Sequential Strategy. Molecules. 2022; 27(3):632. https://doi.org/10.3390/molecules27030632
Chicago/Turabian StyleTorres-Pastor, Miguel Ángel, Claudia Espro, Maurizio Selva, Alvise Perosa, Antonio A. Romero Reyes, Sameh M. Osman, Rafael Luque, and Daily Rodríguez-Padrón. 2022. "Glycerol Valorization towards a Benzoxazine Derivative through a Milling and Microwave Sequential Strategy" Molecules 27, no. 3: 632. https://doi.org/10.3390/molecules27030632
APA StyleTorres-Pastor, M. Á., Espro, C., Selva, M., Perosa, A., Romero Reyes, A. A., Osman, S. M., Luque, R., & Rodríguez-Padrón, D. (2022). Glycerol Valorization towards a Benzoxazine Derivative through a Milling and Microwave Sequential Strategy. Molecules, 27(3), 632. https://doi.org/10.3390/molecules27030632