Surface Enhanced Raman Spectroscopy With Electrodeposited Copper Ultramicro-Wires With/Without Silver Nanostars Decoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Electrochemical Methods
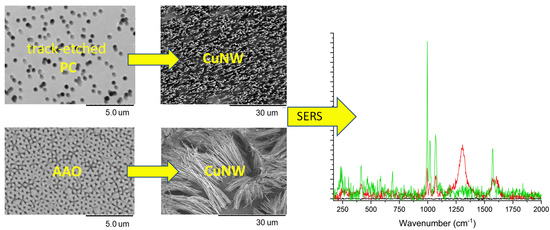
2.3. CuUWs by Polycarbonate Membrane
2.4. CuUWs by Alumina Membrane
2.5. Preparation of Silver Nanostars
2.6. Samples for SERS Analysis
2.7. SERS Measurements
2.8. Electron Microscopy
3. Results and Discussion
3.1. Voltammetric Study of Cu2+ Reduction
3.2. Optimization of CuUWs Deposition in PC Membranes
3.2.1. Template Deposition of CuUWs in Polycarbonate Membranes
3.2.2. Template Deposition of CuUWs in AAO Membranes
3.3. SERS Detection of Benzenethiol on CuUWs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karimian, N.; Ugo, P. Recent advances in sensing and biosensing with arrays of nanoelectrodes. Curr. Opin. Electrochem. 2019, 16, 106–116. [Google Scholar] [CrossRef]
- LaFratta, C.N.; Walt, D.R. Very High Density Sensing Arrays. Chem. Rev. 2008, 108, 614–637. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.M.; Andoni, I.; Choi, E.J.; Fang, L.; Flores-Zuleta, H.; Humphrey, N.J.; Kim, D.-H.; Shin, J.; Youn, H.; Penner, R.M. Sensors Based Upon Nanowires, Nanotubes, and Nanoribbons: 2016–2020. Anal. Chem. 2021, 93, 124–166. [Google Scholar] [CrossRef]
- Habtamu, H.B.; Ugo, P. Miniaturized Enzymatic Biosensor via Biofunctionalization of the Insulator of Nanoelectrode Ensembles. Electroanalysis 2015, 27, 2187–2193. [Google Scholar] [CrossRef]
- Gaetani, C.; Gheno, G.; Borroni, M.; De Wael, K.; Moretto, L.M.; Ugo, P. Nanoelectrode ensemble immunosensing for the electrochemical identification of ovalbumin in works of art. Electrochim. Acta 2019, 312, 72–79. [Google Scholar] [CrossRef]
- Dahlin, A.B. Size Matters: Problems and Advantages Associated with Highly Miniaturized Sensors. Sensors 2012, 12, 3018–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.J.; Jana, N.R. Controlling the Aspect Ratio of Inoragnic Nanorods and Nanowires. Adv. Mater. 2002, 14, 80–83. [Google Scholar] [CrossRef]
- Zalaffi, M.S.; Karimian, N.; Ugo, P. Review-Electrochemical and SERS Sensors for Cultural Heritage Diagnostics and Conservation: Recent Advances and Prospects. J. Electrochem. Soc. 2020, 167, 037548. [Google Scholar] [CrossRef]
- Dahlin, A.B.; Dielacher, B.; Rajendran, P.; Sugihara, K.; Sannomiya, T.; Zenobi-Wong, M.; Voros, J. Electrochemical plasmonic sensors. Anal. Bioanal. Chem. 2012, 402, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-G.; Kang, M.; Kim, S.; Jung, H.S.; Kim, D.-H. 3D-assembled Ag nanowires for use in plasmon-enhanced spectroscopic sensors. Appl. Spectrosc. Rev. 2019, 54, 325–347. [Google Scholar] [CrossRef]
- Possin, G.E. A Method for Forming Very Small Diameter Wires. Rev. Sci. Instrum. 1970, 41, 772. [Google Scholar] [CrossRef]
- Penner, R.M.; Martin, C.R. Preparation and electrochemical characterization of ultramicroelectrode endembles. Anal. Chem. 1987, 59, 2625–2630. [Google Scholar] [CrossRef]
- Martin, C.R. Membrane-based synthesis of nanomaterials. Chem. Mater. 1996, 8, 1739–1746. [Google Scholar] [CrossRef]
- Ugo, P.; Moretto, L.M. Template deposition of metals. In Handbook of Electrochemistry; Zoski, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 678–709. ISBN 978-0-444-51958-0. [Google Scholar] [CrossRef]
- Inguanta, R.; Piazza, S.; Sunseri, C. Novel procedure for the template synthesis of metal nanostructures. Electrochem. Commun. 2008, 10, 506–509. [Google Scholar] [CrossRef]
- Menon, V.P.; Martin, C.R. Fabrication and Evaluation of Nanoelectrode Ensembles. Anal. Chem. 1995, 67, 1920–1928. [Google Scholar] [CrossRef]
- Diggle, W.J.; Downie, C.T.; Goulding, W.C. Anodic oxide films on aluminum. Chem. Rev. 2002, 69, 365–405. [Google Scholar] [CrossRef]
- Sarkar, J.; Khan, G.G.; Basumallick, A. Nanowires: Properties, applications and synthesis via porous aluminium oxide template. Bull. Mater. Sci. 2007, 30, 271–290. [Google Scholar] [CrossRef] [Green Version]
- Sousa, C.T.; Leitao, D.C.; Proenca, M.P.; Ventura, J.; Pereira, A.M.; Araujo, J.P. Nanoporous alumina as templates for multifunctional applications. Appl. Phys. Rev. 2014, 1, 031102. [Google Scholar] [CrossRef]
- Giallongo, G.; Durante, C.; Pilot, R.; Garoli, D.; Bozio, R.; Romanato, F.; Gennaro, A.; Rizzi, G.A.; Granozzi, G. Growth and optical properties of silver nanostructures obtained on connected anodic aluminum oxide templates. Nanotechnology 2012, 23, 325604. [Google Scholar] [CrossRef] [PubMed]
- Whitney, T.M.; Jiang, J.S.; Searson, P.C.; Chien, C.L. Fabrication and magnetic-properties of arrays of metallic nanowires. Science 1993, 261, 1316–1319. [Google Scholar] [CrossRef] [PubMed]
- Schonenberger, C.; Van der Zande, B.M.I.; Fokkink, L.G.J.; Henny, M.; Schmid, C.; Kruger, M.; Bachtold, A.; Huber, R.; Birk, H.; Staufer, U. Template synthesis of nanowires in porous polycarbonate membranes: Electrochemistry and morphology. J. Phys. Chem. B 1997, 101, 5497–5505. [Google Scholar] [CrossRef]
- Blanco, S.; Vargas, R.; Mostany, J.; Borrás, C.; Scharifker, B.R. Modeling the Growth of Nanowire Arrays in Porous Membrane Templates. J. Electrochem. Soc. 2014, 161, E3341–E3347. [Google Scholar] [CrossRef]
- Davydov, A.D.; Volgin, V.M. Template electrodeposition of metals. Review. Russ. J. Electrochem. 2016, 52, 806–831. [Google Scholar] [CrossRef]
- Yu, S.; Li, N.; Wharton, J.; Martin, C.R. Nano wheat fields prepared by plasma-etching gold nanowire-containing membranes. Nano Lett. 2003, 3, 815–818. [Google Scholar] [CrossRef]
- Gasparac, R.; Taft, B.J.; Lapierre-Devlin, M.A.; Lazareck, A.D.; Xu, J.M.; Kelley, S.O. Ultrasensitive electrocatalytic DNA detection at two- and three-dimensional nanoelectrodes. J. Am. Chem. Soc. 2004, 126, 12270–12271. [Google Scholar] [CrossRef] [PubMed]
- De Leo, M.; Kuhn, A.; Ugo, P. 3D-ensembles of gold nanowires: Preparation, characterization and electroanalytical peculiarities. Electroanalysis 2007, 19, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Yan, P.; Sun, K.; Wirk, D.W. Tailor-made gold brush nanoelectrode ensembles modified with L-cysteine for the detection of daunorubicine. Electrochim. Acta 2008, 53, 8144–8148. [Google Scholar] [CrossRef]
- Beluomini, M.A.; Karimian, N.; Stradiotto, N.R.; Ugo, P. Tailor-made 3D-nanoelectrode ensembles modified with molecularly imprinted poly(o-phenylenediamine) for the sensitive detection of L-arabitol. Sens. Actuators B-Chem. 2019, 284, 250–257. [Google Scholar] [CrossRef]
- Gao, T.; Meng, G.W.; Wang, Y.W.; Sun, S.H.; Zhang, L. Electrochemical synthesis of copper nanowires. J. Phys.-Condens. Matter 2002, 14, 355–363. [Google Scholar] [CrossRef]
- Ganapathi, A.; Swaminathan, P.; Neelakantan, L. Anodic Aluminum Oxide Template Assisted Synthesis of Copper Nanowires using a Galvanic Displacement Process for Electrochemical Denitrification. ACS Appl. Nano Mater. 2019, 2, 5981–5988. [Google Scholar] [CrossRef]
- Stortini, A.M.; Moretto, L.M.; Mardegan, A.; Ongaro, M.; Ugo, P. Arrays of copper nanowire electrodes: Preparation, characterization and application as nitrate sensor. Sens. Actuators B Chem. 2015, 207, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Stortini, A.M.; Fabris, S.; Saorin, G.; Falzacappa, E.V.; Moretto, L.M.; Ugo, P. Plasma Activation of Copper Nanowires Arrays for Electrocatalytic Sensing of Nitrate in Food and Water. Nanomaterials 2019, 9, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patella, B.; Russo, R.R.; O’Riordan, A.; Aiello, G.; Sunseri, C.; Inguanta, R. Copper nanowire array as highly selective electrochemical sensor of nitrate ions in water. Talanta 2021, 221, 121643. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Bantz, K.C.; Meyer, A.F.; Wittenberg, N.J.; Im, H.; Kurtulus, O.; Lee, S.H.; Lindquist, N.C.; Oh, S.-H.; Haynes, C.L. Recent progress in SERS biosensing. Phys. Chem. Chem. Phys. 2011, 13, 11551–11567. [Google Scholar] [CrossRef] [PubMed]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Chourpa, I.; Lei, F.H.; Dubois, P.; Manfait, M.; Sockalingum, G.D. Intracellular applications of analytical SERS spectroscopy and multispectral imaging. Chem. Soc. Rev. 2008, 37, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Lussier, F.; Brule, T.; Vishwakarma, M.; Das, T.; Spatz, J.P.; Masson, J.-F. Dynamic-SERS Optophysiology: A Nanosensor for Monitoring Cell Secretion Events. Nano Lett. 2016, 16, 3866–3871. [Google Scholar] [CrossRef]
- Lee, S.J.; Baik, J.M.; Moskovits, M. Polarization-Dependent Surface-Enhanced Raman Scattering from a Silver-Nanoparticle-Decorated Single Silver Nanowire. Nano Lett. 2008, 8, 3244–3247. [Google Scholar] [CrossRef]
- Liu, S.; Chen, N.; Li, L.; Pang, F.; Chen, Z.; Wang, T. Fabrication of Ag/Au core-shell nanowire as a SERS substrate. Opt. Mater. 2013, 35, 690–692. [Google Scholar] [CrossRef]
- Du, Y.; Shi, L.; He, T.; Sun, X.; Mo, Y. SERS enhancement dependence on the diameter and aspect ratio of silver-nanowire array fabricated by anodic aluminium oxide template. Appl. Surf. Sci. 2008, 255, 1901–1905. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, X.; Dai, S.; Du, Z. Single-crystal silver nanowires: Preparation and Surface-enhanced Raman Scattering (SERS) property. Mater. Lett. 2009, 63, 2570–2573. [Google Scholar] [CrossRef] [Green Version]
- Zalaffi, M.S.; Litti, L.; Canton, P.; Meneghetti, M.; Moretto, L.M.; Ugo, P. Preparation and characterization of Ag-nanostars@Au-nanowires hierarchical nanostructures for highly sensitive surface enhanced Raman spectroscopy. Nano Express 2020, 1, 020006. [Google Scholar] [CrossRef]
- Halouzka, V.; Halouzkova, B.; Jirovsky, D.; Hemzal, D.; Ondra, P.; Siranidi, E.; Kontos, A.G.; Falaras, P.; Hrbac, J. Copper nanowire coated carbon fibers as efficient substrates for detecting designer drugs using SERS. Talanta 2017, 165, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; He, S.-Y.; Tuan, H.-Y. Self-Seeded Growth of Five-Fold Twinned Copper Nanowires: Mechanistic Study, Characterization and SERS Applications. Langmuir 2014, 30, 602–610. [Google Scholar] [CrossRef]
- Xu, D.; Dong, Z.; Sun, J.-L. Fabrication of copper nanowires by a solid-state ionics method and their surface enhanced Raman scattering effect. Mater. Lett. 2013, 92, 143–146. [Google Scholar] [CrossRef]
- Ugo, P.; Moretto, L.M.; Mazzocchin, G.A. Ion-exchange voltammetry of copper ions in chloride media at glassy carbon electrodes modified with polycationic ionomers. Anal. Chim. Acta 1993, 273, 229–236. [Google Scholar] [CrossRef]
- Garcia-Leis, A.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Silver Nanostars with High SERS Performance. J. Phys. Chem. C 2013, 117, 12397. [Google Scholar] [CrossRef]
- Zalaffi, M.S.; Agostinelli, I.; Karimian, N.; Ugo, P. Ag-Nanostars for the Sensitive SERS Detection of Dyes in Artistic Cross-Sections—Madonna della Misericordia of the National Gallery of Parma: A Case Study. Heritage 2020, 3, 1344–1359. [Google Scholar] [CrossRef]
- Milchev, A.; Zapryanova, T. Nucleation and growth of copper under combined charge transfer and diffusion limitations: Part I. Electrochim. Acta 2006, 51, 2926–2933. [Google Scholar] [CrossRef]
- Milchev, A.; Zapryanova, T. Nucleation and growth of copper under combined charge transfer and diffusion limitations - Part II. Electrochim. Acta 2006, 51, 4916–4921. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L. Electrochemical Methods Fundamentalsand Applications, 2nd ed.; Wiley: New York, NY, USA, 2001; ISBN 0-471-04372-9. [Google Scholar] [CrossRef]
- Vydra, F.; Stulik, K.; Julakova, E. Electrochemical stripping analysis. In Ellis Horwood Series in Analytical Chemistry; Red, H.P., Ed.; Prentice Hall: New York, NY, USA, 1976; ISBN 10: 085312017X/ISBN 13: 9780853120179. [Google Scholar]
- Moretto, L.M.; Mazzocchin, G.A.; Ugo, P. Electroanalytical study on the ion-exchange voltammetric behaviour of Hg(II) at Tosflex(R)-coated glassy carbon electrodes. J. Electroanal. Chem. 1997, 427, 113–121. [Google Scholar] [CrossRef]
- Joo, T.H.; Kim, M.S.; Kim, K. Surface-enhanced Raman-scattering of benzenethiol in silver sol. J. Raman Spectrosc. 1987, 18, 57–60. [Google Scholar] [CrossRef]
- McFarland, A.D.; Young, M.A.; Dieringer, J.A.; Van Duyne, R.P. Wavelength-scanned surface-enhanced Raman excitation spectroscopy. J. Phys. Chem. B 2005, 109, 11279–11285. [Google Scholar] [CrossRef] [PubMed]
- Zamuner, M.; Talaga, D.; Deiss, F.; Guieu, V.; Kuhn, A.; Ugo, P.; Sojic, N. Fabrication of a Macroporous Microwell Array for Surface-Enhanced Raman Scattering. Adv. Funct. Mater. 2009, 19, 3129–3135. [Google Scholar] [CrossRef]
- Aggarwal, R.L.; Farrar, L.W.; Diebold, E.D.; Polla, D.L. Measurement of the absolute Raman scattering cross section of the 1584 cm−1 band of benzenethiol and the surface-enhanced Raman scattering cross section enhancement factor for femtosecond laser-nanostructured substrates. J. Raman Spectrosc. 2009, 40, 1331–1333. [Google Scholar] [CrossRef]
- Fontana, J.; Livenerec, J.; Bezares, F.J.; Caldwell, J.D.; Rendell, R.; Ratna, B.R. Large surface-enhanced Raman scattering from self-assembled gold nanosphere monolayers. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- McCreery, R.I. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Mardegan, A.; Kamath, R.; Sharma, S.; Scopece, P.; Ugo, P.; Madou, M. Optimization of Carbon Electrodes Derived from Epoxy-based Photoresist. J. Electrochem. Soc. 2013, 160, B132–B137. [Google Scholar] [CrossRef] [Green Version]
- Guieu, V.; Lagugne-Labarthet, F.; Servant, L.; Talaga, D.; Sojic, N. Ultrasharp Optical Fiber Nanoprobe Array for Raman Local Enhancement Imaging. Small 2008, 4, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]









| [CuSO4] (M) | Scan Rate (mV/s) | Epc vs. Cu (V) | Epc vs. SHE (V) | Ipc (mA) | Epa vs. Cu (V) | Epa vs. SHE (V) | Ipa (mA) | Apa (mC) |
|---|---|---|---|---|---|---|---|---|
| 0.01 | 10 | −0.290 | −0.010 | −0.12 | 0.130 | 0.410 | 1.10 | 8.0 |
| 20 | −0.350 | −0.070 | −0.20 | 0.135 | 0.415 | 1.48 | 4.0 | |
| 40 | −0.380 | −0.100 | −0.30 | 0.138 | 0.418 | 1.90 | 3.5 | |
| 80 | −0.535 | −0.255 | −0.40 | 0.140 | 0.420 | 2.10 | 1.7 | |
| 0.05 | 10 | −0.280 | −0.020 | −0.68 | 0.132 | 0.432 | 3.50 | 30 |
| 20 | −0.310 | −0.010 | −0.96 | 0.140 | 0.439 | 4.58 | 18.0 | |
| 40 | −0.340 | −0.040 | −1.50 | 0.156 | 0.456 | 5.80 | 12.0 | |
| 80 | −0.370 | −0.070 | −1.90 | 0.167 | 0.467 | 6.90 | 8.8 | |
| 0.30 | 10 | −0.215 | 0.105 | −4.7 | 0.285 | 0.605 | 14.5 | 220 |
| 20 | −0.250 | 0.070 | −6.3 | 0.305 | 0.625 | 15.7 | 130 | |
| 40 | −0.300 | 0.020 | −85 | 0.320 | 0.640 | 17.9 | 76 | |
| 80 | −0.400 | −0.080 | −11.8 | 0.360 | 0.680 | 19.5 | 50 |
| Nanomaterial | Specific Surface 1 (nm2/cm2) | Surface Coverage 2 (Molecules/cm2) | N3 (Molecules) | ISERS4 (Counts/Watt × s) | EF 5 |
|---|---|---|---|---|---|
| CuUWs | 1.2 × 1015 | 5.7 × 1015 | 2.5 × 108 | 7.5 × 104 | 0.7 × 104 |
| AgNS@CuNW | 1.80 × 1015 | 8.2 × 1015 | 3.7 × 108 | 25.0 × 104 | 1.6 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longoni, M.; Zalaffi, M.S.; de Ferri, L.; Stortini, A.M.; Pojana, G.; Ugo, P. Surface Enhanced Raman Spectroscopy With Electrodeposited Copper Ultramicro-Wires With/Without Silver Nanostars Decoration. Nanomaterials 2021, 11, 518. https://doi.org/10.3390/nano11020518
Longoni M, Zalaffi MS, de Ferri L, Stortini AM, Pojana G, Ugo P. Surface Enhanced Raman Spectroscopy With Electrodeposited Copper Ultramicro-Wires With/Without Silver Nanostars Decoration. Nanomaterials. 2021; 11(2):518. https://doi.org/10.3390/nano11020518
Chicago/Turabian StyleLongoni, Margherita, Maria Sole Zalaffi, Lavinia de Ferri, Angela Maria Stortini, Giulio Pojana, and Paolo Ugo. 2021. "Surface Enhanced Raman Spectroscopy With Electrodeposited Copper Ultramicro-Wires With/Without Silver Nanostars Decoration" Nanomaterials 11, no. 2: 518. https://doi.org/10.3390/nano11020518
APA StyleLongoni, M., Zalaffi, M. S., de Ferri, L., Stortini, A. M., Pojana, G., & Ugo, P. (2021). Surface Enhanced Raman Spectroscopy With Electrodeposited Copper Ultramicro-Wires With/Without Silver Nanostars Decoration. Nanomaterials, 11(2), 518. https://doi.org/10.3390/nano11020518