Thermoelectric Energy Harvesting from Single-Walled Carbon Nanotube Alkali-Activated Nanocomposites Produced from Industrial Waste Materials
Abstract
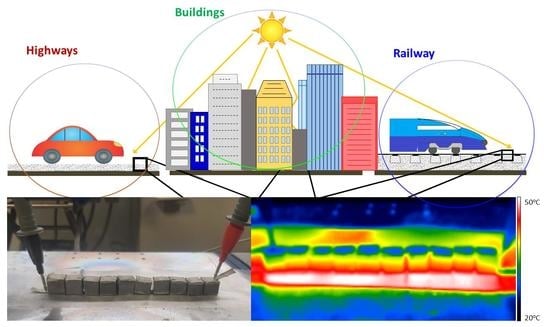
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Alkali-Activated Nanocomposites’ Fabrication
2.2.2. Electrical and Thermoelectric Properties Characterization
2.2.3. Thermoelectric Generator Characterization
2.2.4. Mechanical and Microstructural Properties Characterization
3. Results and Discussion
3.1. Thermoelectric Properties
3.2. Power Output of the Thermoelectric Generator
3.3. Stability of the Thermoelectric Generator and Performance over Time
3.4. Mechanical Properties
3.5. Microstructure of 1.0 wt.% SWCNT Nanocomposites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Part, W.K.; Ramli, M.; Cheah, C.B. An Overview on the Influence of Various Factors on the Properties of Geopolymer Concrete Derived from Industrial Byproducts. In Handbook of Low Carbon Concrete; Elsevier: Amsterdam, The Netherlands, 2017; pp. 263–334. ISBN 9780128045244. [Google Scholar]
- Van Deventer, J. Progress in the Adoption of Geopolymer Cement. In Handbook of Low Carbon Concrete; Elsevier: Amsterdam, The Netherlands, 2017; pp. 217–262. ISBN 9780128045244. [Google Scholar]
- Zhang, Z.; Yang, T.; Wang, H. Alkali-Activated Cement (AAC) From Fly Ash and High-Magnesium Nickel Slag. In Handbook of Low Carbon Concrete; Elsevier: Amsterdam, The Netherlands, 2017; pp. 357–374. ISBN 9780128045244. [Google Scholar]
- Provis, J.L.; van Deventer, J.S. Geopolymers and Other Alkali-Activated Materials. In Lea’s Chemistry of Cement and Concrete; Elsevier: Amsterdam, The Netherlands, 2019; pp. 779–805. ISBN 9780081007730. [Google Scholar]
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Rees, G.J.; Hanna, J.V.; van Deventer, J.S.; Provis, J.L. Phase evolution of C-(N)-A-S-H/N-A-S-H gel blends investigated via alkali-activation of synthetic calcium aluminosilicate precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef] [Green Version]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Athira, V.S.; Charitha, V.; Athira, G.; Bahurudeen, A. Agro-waste ash based alkali-activated binder: Cleaner production of zero cement concrete for construction. J. Clean. Prod. 2021, 286, 125429. [Google Scholar] [CrossRef]
- Provis, J.L. Activating solution chemistry for geopolymers. In Geopolymers; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9781845694494. [Google Scholar]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Abdulkareem, M.; Havukainen, J.; Nuortila-Jokinen, J.; Horttanainen, M. Environmental and economic perspective of waste-derived activators on alkali-activated mortars. J. Clean. Prod. 2021, 280, 124651. [Google Scholar] [CrossRef]
- Vinai, R.; Soutsos, M. Production of sodium silicate powder from waste glass cullet for alkali activation of alternative binders. Cem. Concr. Res. 2019, 116, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Davoodabadi, M.; Liebscher, M.; Hampel, S.; Sgarzi, M.; Rezaie, A.B.; Wolf, D.; Cuniberti, G.; Mechtcherine, V.; Yang, J. Multi-walled carbon nanotube dispersion methodologies in alkaline media and their influence on mechanical reinforcement of alkali-activated nanocomposites. Compos. Part B 2021, 209, 108559. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.; Jäger, C.; Brouwers, H.; Kühne, H.-C. Sulfuric acid resistance of one-part alkali-activated mortars. Cem. Concr. Res. 2018, 109, 54–63. [Google Scholar] [CrossRef]
- Novais, R.M.; Carvalheiras, J.; Senff, L.; Lacasta, A.M.; Cantalapiedra, I.R.; Giro-Paloma, J.; Seabra, M.P.; Labrincha, J.A. Multifunctional cork—alkali-activated fly ash composites: A sustainable material to enhance buildings’ energy and acoustic performance. Energy Build. 2020, 210, 109739. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Li, W.; Hu, Y.; Zhou, J.L.; Tam, V.W. Review on designs and properties of multifunctional alkali-activated materials (AAMs). Constr. Build. Mater. 2019, 200, 474–489. [Google Scholar] [CrossRef]
- Vlachakis, C.; Perry, M.; Biondi, L. Self-Sensing Alkali-Activated Materials: A Review. Minerals 2020, 10, 885. [Google Scholar] [CrossRef]
- Biondi, L.; Perry, M.; McAlorum, J.; Vlachakis, C.; Hamilton, A. Geopolymer-based moisture sensors for reinforced concrete health monitoring. Sens. Actuators B 2020, 309, 127775. [Google Scholar] [CrossRef]
- Rovnaník, P.; Kusák, I.; Bayer, P.; Schmid, P.; Fiala, L. Comparison of electrical and self-sensing properties of Portland cement and alkali-activated slag mortars. Cem. Concr. Res. 2019, 118, 84–91. [Google Scholar] [CrossRef]
- Saafi, M.; Piukovics, G.; Ye, J. Hybrid graphene/geopolymeric cement as a superionic conductor for structural health monitoring applications. Smart Mater. Struct. 2016, 25, 105018. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Hunpratub, S.; Thongbai, P.; Maensiri, S.; Sata, V.; Chindaprasirt, P. Effects of NaOH concentrations on physical and electrical properties of high calcium fly ash geopolymer paste. Cem. Concr. Compos. 2014, 45, 9–14. [Google Scholar] [CrossRef]
- Saafi, M.; Gullane, A.; Huang, B.; Sadeghi, H.; Ye, J.; Sadeghi, F. Inherently multifunctional geopolymeric cementitious composite as electrical energy storage and self-sensing structural material. Compos. Struct. 2018, 201, 766–778. [Google Scholar] [CrossRef] [Green Version]
- Kočí, V.; Petříková, M.; Fořt, J.; Fiala, L.; Černý, R. Preparation of self-heating alkali-activated materials using industrial waste products. J. Clean. Prod. 2020, 260, 121116. [Google Scholar] [CrossRef]
- Bi, S.; Liu, M.; Shen, J.; Hu, X.M.; Zhang, L. Ultrahigh Self-Sensing Performance of Geopolymer Nanocomposites via Unique Interface Engineering. ACS Appl. Mater. Interfaces 2017, 9, 12851–12858. [Google Scholar] [CrossRef]
- Zhong, J.; Zhou, G.-X.; He, P.-G.; Yang, Z.-H.; Jia, D.-C. 3D printing strong and conductive geo-polymer nanocomposite structures modified by graphene oxide. Carbon 2017, 117, 421–426. [Google Scholar] [CrossRef]
- Zhang, Y.J.; He, P.Y.; Zhang, Y.X.; Chen, H. A novel electroconductive graphene/fly ash-based geopolymer composite and its photocatalytic performance. Chem. Eng. J. 2018, 334, 2459–2466. [Google Scholar] [CrossRef]
- Rovnaník, P.; Kusák, I.; Bayer, P.; Schmid, P.; Fiala, L. Electrical and Self-Sensing Properties of Alkali-Activated Slag Composite with Graphite Filler. Materials 2019, 12, 1616. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, K.J.D.; Bolton, M.J. Electrical and mechanical properties of aluminosilicate inorganic polymer composites with carbon nanotubes. J. Mater. Sci. 2009, 44, 2851–2857. [Google Scholar] [CrossRef]
- Kusak, I.; Lunak, M. On the effect of addition of carbon nanotubes on the electric conductivity of alkali-activated slag mortars. IOP Conf. Ser. Mater. Sci. Eng. 2017, 246, 12044. [Google Scholar] [CrossRef] [Green Version]
- Saafi, M.; Andrew, K.; Tang, P.L.; McGhon, D.; Taylor, S.; Rahman, M.; Yang, S.; Zhou, X. Multifunctional properties of carbon nanotube/fly ash geopolymeric nanocomposites. Constr. Build. Mater. 2013, 49, 46–55. [Google Scholar] [CrossRef]
- Zoui, M.A.; Bentouba, S.; Stocholm, J.G.; Bourouis, M. A Review on Thermoelectric Generators: Progress and Applications. Energies 2020, 13, 3606. [Google Scholar] [CrossRef]
- Zabihi, N.; Saafi, M. Recent Developments in the Energy Harvesting Systems from Road Infrastructures. Sustainability 2020, 12, 6738. [Google Scholar] [CrossRef]
- Tzounis, L.; Liebscher, M.; Fuge, R.; Leonhardt, A.; Mechtcherine, V. P- and n-type thermoelectric cement composites with CVD grown p- and n-doped carbon nanotubes: Demonstration of a structural thermoelectric generator. Energy Build. 2019, 191, 151–163. [Google Scholar] [CrossRef]
- Ghosh, S.; Harish, S.; Ohtaki, M.; Saha, B.B. Thermoelectric figure of merit enhancement in cement composites with graphene and transition metal oxides. Mater. Today Energy 2020, 18, 100492. [Google Scholar] [CrossRef]
- Cai, J.; Tan, J.; Li, X. Thermoelectric behaviors of fly ash and metakaolin based geopolymer. Constr. Build. Mater. 2020, 237, 117757. [Google Scholar] [CrossRef]
- Li, J.; Tay, B.W.Y.; Lei, J.; Yang, E.-H. Experimental investigation of Seebeck effect in metakaolin-based geopolymer. Constr. Build. Mater. 2021, 272, 121615. [Google Scholar] [CrossRef]
- Liu, X.; Jani, R.; Orisakwe, E.; Johnston, C.; Chudzinski, P.; Qu, M.; Norton, B.; Holmes, N.; Kohanoff, J.; Stella, L.; et al. State of the art in composition, fabrication, characterization, and modeling methods of cement-based thermoelectric materials for low-temperature applications. Renew. Sustain. Energy Rev. 2021, 137, 110361. [Google Scholar] [CrossRef]
- Tzounis, L. Synthesis and Processing of Thermoelectric Nanomaterials, Nanocomposites, and Devices. In Nanomaterials Synthesis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 295–336. ISBN 9780128157510. [Google Scholar]
- Champier, D. Thermoelectric generators: A review of applications. Energy Convers. Manag. 2017, 140, 167–181. [Google Scholar] [CrossRef]
- Smits, F.M. Measurement of Sheet Resistivities with the Four-Point Probe. Bell Syst. Tech. J. 1958, 37, 711–718. [Google Scholar] [CrossRef]
- Wen, S.; Chung, D. Origin of the thermoelectric behavior of steel fiber cement paste. Cem. Concr. Res. 2002, 32, 821–823. [Google Scholar] [CrossRef]
- Krause, B.; Konidakis, I.; Arjmand, M.; Sundararaj, U.; Fuge, R.; Liebscher, M.; Hampel, S.; Klaus, M.; Serpetzoglou, E.; Stratakis, E.; et al. Nitrogen-Doped Carbon Nanotube/Polypropylene Composites with Negative Seebeck Coefficient. J. Compos. Sci. 2020, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Ding, Y.; Qiu, Y.; Cai, K.; Yao, Q.; Song, H.; Tong, L.; He, J.; Chen, L. Good Performance and Flexible PEDOT:PSS/Cu2Se Nanowire Thermoelectric Composite Films. ACS Appl. Mater. Interfaces 2019, 11, 12819–12829. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, H.; Zhao, D.; Zhang, F.; Hu, D.; Jiao, F.; Qin, L.; Linseis, V.; Fabiano, S.; Crispin, X.; et al. High Thermoelectric Performance in n-Type Perylene Bisimide Induced by the Soret Effect. Adv. Mater. 2020, 32, e2002752. [Google Scholar] [CrossRef] [PubMed]
- Rudyak, V.Y.; Minakov, A.V.; Pryazhnikov, M.I. Preparation, characterization, and viscosity studding the single-walled carbon nanotube nanofluids. J. Mol. Liq. 2021, 329, 115517. [Google Scholar] [CrossRef]
- Zhang, L.; Harima, Y.; Imae, I. Highly improved thermoelectric performances of PEDOT:PSS/SWCNT composites by solvent treatment. Org. Electron. 2017, 51, 304–307. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, Z.; Wang, B. The exfoliation of SWCNT bundles examined by simultaneous Raman scattering and photoluminescence spectroscopy. Carbon 2009, 47, 3529–3537. [Google Scholar] [CrossRef]
- Alafogianni, P.; Dassios, K.; Tsakiroglou, C.D.; Matikas, T.E.; Barkoula, N.M. Effect of CNT addition and dispersive agents on the transport properties and microstructure of cement mortars. Constr. Build. Mater. 2019, 197, 251–261. [Google Scholar] [CrossRef]
- Liebscher, M.; Gärtner, T.; Tzounis, L.; Mičušík, M.; Pötschke, P.; Stamm, M.; Heinrich, G.; Voit, B. Influence of the MWCNT surface functionalization on the thermoelectric properties of melt-mixed polycarbonate composites. Compos. Sci. Technol. 2014, 101, 133–138. [Google Scholar] [CrossRef]









| CNT Content | G/D | Outer Mean Diameter | Length | Aspect Ratio | Specific Surface Area | Metal Impurities |
|---|---|---|---|---|---|---|
| wt.% | - | nm | µm | - | m2·g−1 | wt.% |
| ≥80 | >90 | 1.6 ± 0.4 | >5 | 3000 | 1000 | ≤15 |
| SWCNT | SWCNT | SDBS 10% | W/S | DI-Water | Fly Ash | GGBS | Sodium Disilicate |
|---|---|---|---|---|---|---|---|
| wt.% | mg | g | - | g | g | g | g |
| 0.1 | 34 | 0.34 | 0.45 | 16.50 | 24 | 10 | 6 |
| 0.5 | 170 | 1.70 | 0.45 | 16.50 | 24 | 10 | 6 |
| 1.0 | 340 | 3.40 | 0.45 | 16.50 | 24 | 10 | 6 |
| SWCNT Concentration | Electrical Conductivity | Seebeck Coefficient | Power Factor |
|---|---|---|---|
| SWCNT | σ | S (−ΔV/ΔT) | PF (σ·S2) |
| wt.% | S·m−1 | μV·K−1 | μW·m−1·K−2 |
| 0.1 | 9.42 × 102 ± 8.9 × 10−1 | 17.6 ± 0.8 | 0.292 ± 0.05 |
| 0.5 | 1.23 × 103 ± 5.1 × 100 | 16.6 ± 0.8 | 0.340 ± 0.04 |
| 1.0 | 1.66 × 103 ± 5.1 × 100 | 15.8 ± 0.8 | 0.414 ± 0.04 |
| ΔT (K) | Pdensity (nW m−2) | Pmax (μW) | VTEP (mV) |
|---|---|---|---|
| 15 | 23 | 0.085 | 2.1 |
| 30 | 171 | 0.251 | 3.8 |
| 60 | 372 | 0.695 | 7.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davoodabadi, M.; Vareli, I.; Liebscher, M.; Tzounis, L.; Sgarzi, M.; Paipetis, A.S.; Yang, J.; Cuniberti, G.; Mechtcherine, V. Thermoelectric Energy Harvesting from Single-Walled Carbon Nanotube Alkali-Activated Nanocomposites Produced from Industrial Waste Materials. Nanomaterials 2021, 11, 1095. https://doi.org/10.3390/nano11051095
Davoodabadi M, Vareli I, Liebscher M, Tzounis L, Sgarzi M, Paipetis AS, Yang J, Cuniberti G, Mechtcherine V. Thermoelectric Energy Harvesting from Single-Walled Carbon Nanotube Alkali-Activated Nanocomposites Produced from Industrial Waste Materials. Nanomaterials. 2021; 11(5):1095. https://doi.org/10.3390/nano11051095
Chicago/Turabian StyleDavoodabadi, Maliheh, Ioanna Vareli, Marco Liebscher, Lazaros Tzounis, Massimo Sgarzi, Alkiviadis S. Paipetis, Jian Yang, Gianaurelio Cuniberti, and Viktor Mechtcherine. 2021. "Thermoelectric Energy Harvesting from Single-Walled Carbon Nanotube Alkali-Activated Nanocomposites Produced from Industrial Waste Materials" Nanomaterials 11, no. 5: 1095. https://doi.org/10.3390/nano11051095