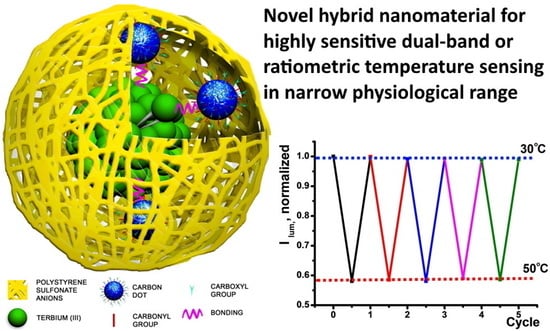
Single Excited Dual Band Luminescent Hybrid Carbon Dots-Terbium Chelate Nanothermometer
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Synthesis, Characterization, and Sensing Properties of CDs
3.2. Synthesis, Characterization, and Sensing Properties of PSS-[TbL]
3.3. Interactions between CDs and [TbL]+ in the DMF Solutions
3.4. Synthesis and Characterization of PSS-{CDs-[TbL]} Colloids
3.5. Sensing Properties of PSS-{CDs-[TbL]}
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katagiri, S.; Hasegawa, Y.; Wada, Y.; Yanagida, S. Thermo-sensitive Luminescence Based on the Back Energy Transfer in Terbium(III) Complexes. Chem. Lett. 2004, 33, 1438–1439. [Google Scholar] [CrossRef]
- Runowski, M.; Woźny, P.; Martín, I.R. Optical pressure sensing in vacuum and high-pressure ranges using lanthanide-based luminescent thermometer–manometer. J. Mater. Chem. C 2021, 9, 4643–4651. [Google Scholar] [CrossRef]
- Zheng, T.; Qiu, X.; Zhou, L.; Runowski, M.; Lis, S.; Du, P.; Luo, L. Multiple ratiometric nanothermometry operating with Stark thermally and non-thermally-coupled levels in upconverting Y2−xMoO6:xEr3+ nanoparticles. J. Alloys Compd. 2021, 864, 158891. [Google Scholar] [CrossRef]
- Takei, Y.; Arai, S.; Murata, A.; Takabayashi, M.; Oyama, K.; Ishiwata, S.; Takeoka, S.; Suzuki, M. A Nanoparticle-Based Ratiometric and Self-Calibrated Fluorescent Thermometer for Single Living Cells. ACS Nano 2014, 8, 198–206. [Google Scholar] [CrossRef]
- Vanden Bussche, F.; Kaczmarek, A.M.; Schmidt, J.; Stevens, C.V.; Van Der Voort, P. Lanthanide grafted phenanthroline-polymer for physiological temperature range sensing. J. Mater. Chem. C 2019, 7, 10972–10980. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Zhen, X.; Zeng, Z.; Li, J.; Xie, C.; Miao, Q.; Chen, J.; Chen, P.; Pu, K. A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 2019, 10, 2064. [Google Scholar] [CrossRef]
- Palner, M.; Pu, K.; Shao, S.; Rao, J. Semiconducting Polymer Nanoparticles with Persistent Near-Infrared Luminescence for In Vivo Optical Imaging Angewandte. Angew. Chem. 2015, 127, 11639–11642. [Google Scholar] [CrossRef]
- Jiang, L.; Bai, H.; Liu, L.; Lv, F.; Ren, X.; Wang, S. Zuschriften Photodynamic Therapy Luminescent, Oxygen-Supplying, Hemoglobin-Linked Conjugated Polymer Nanoparticles for Photodynamic Therapy Zuschriften Angewandte. Angew. Chem. 2019, 131, 10770–10775. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Zhang, C.-H.; Jiang, J.; Gao, Y.; Zhang, P.; Zeng, R.; Chen, J. Zero-crosstalk and color-specific photoswitching of dual-emissive polymer nanoparticles for multiple applications. Dye Pigment 2021, 191, 109370. [Google Scholar] [CrossRef]
- Zairov, R.R.; Dovzhenko, A.P.; Sapunova, A.S.; Voloshina, A.D.; Tatarinov, D.A.; Nizameev, I.R.; Gubaidullin, A.T.; Petrov, K.A.; Enrichi, F.; Vomiero, A.; et al. Dual red-NIR luminescent Eu Yb heterolanthanide nanoparticles as promising basis for cellular imaging and sensing. Mater. Sci. Eng. C 2019, 105, 110057. [Google Scholar] [CrossRef]
- Fedorenko, S.; Gilmanova, D.; Mukhametshina, A.; Nizameev, I.; Kholin, K.; Akhmadeev, B.; Voloshina, A.; Sapunova, A.; Kuznetsova, S.; Daminova, A.; et al. Silica nanoparticles with dual visible–NIR luminescence affected by silica confinement of Tb(III) and Yb(III) complexes for cellular imaging application. J. Mater. Sci. 2019, 54, 9140–9154. [Google Scholar] [CrossRef]
- Podyachev, S.N.; Zairov, R.R.; Mustafina, A.R. 1,3-Diketone Calix[4]arene Derivatives-A New Type of Versatile Ligands for Metal Complexes and Nanoparticles. Molecules 2021, 26, 1214. [Google Scholar] [CrossRef] [PubMed]
- Sukhishvili, S.A.; Kharlampieva, E.; Izumrudov, V. Where polyelectrolyte multilayers and polyelectrolyte complexes meet. Macromolecules 2006, 39, 8873–8881. [Google Scholar] [CrossRef]
- Radulescu, A.; Murmiliuk, A.; Filippov, S.K.; Rud, O.; Košovan, P. Reversible multilayered vesicle-like structures with fluid hydrophobic and interpolyelectrolyte layers. J. Colloid Interface Sci. 2021, 599, 313–325. [Google Scholar]
- Kudryavtseva, V.; Boi, S.; Read, J.; Gould, D.; Szewczyk, P.K.; Stachewicz, U.; Kiryukhin, M.V.; Pastorino, L.; Sukhorukov, G.B. Micro-sized “pelmeni”—A universal microencapsulation approach overview. Mater. Des. 2021, 202, 109527. [Google Scholar] [CrossRef]
- Mauser, T.; Déjugnat, C.; Sukhorukov, G.B. Balance of Hydrophobic and Electrostatic Forces in the pH Response of Weak Polyelectrolyte Capsules. J. Phys. Chem. B 2006, 110, 20246–20253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sharma, M.; Berezin, O.; Zuckerman, D.; Berezin, M.Y. Nanothermometry: From Microscopy to Thermal Treatments. ChemPhysChem 2016, 17, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bednarkiewicz, A.; Marciniak, L.; Carlos, L.D.; Jaque, D. Standardizing luminescence nanothermometry for biomedical applications. Nanoscale 2020, 12, 14405–14421. [Google Scholar] [CrossRef]
- Carattino, A.; Caldarola, M.; Orrit, M. Gold Nanoparticles as Absolute Nanothermometers. Nano Lett. 2018, 18, 874–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brites, C.D.S.; Balabhadra, S.; Carlos, L.D. Lanthanide-based thermometers: At the cutting-edge of luminescence thermometry. Adv. Opt. Mater. 2019, 7, 1801239. [Google Scholar] [CrossRef] [Green Version]
- del Rosal, B.; Ximendes, E.; Rocha, U.; Jaque, D. In Vivo Luminescence Nanothermometry: From Materials to Applications. Adv. Opt. Mater. 2017, 5, 1600508. [Google Scholar] [CrossRef]
- Arai, S.; Takeoka, S.; Ishiwata, S.I.; Suzuki, M.; Sato, H. Facilely fabricated luminescent nanoparticle thermosensor for real-time microthermography in living animals. ACS Sens. 2016, 1, 1222–1227. [Google Scholar]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon Dots for Multiphoton Bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Mahajan, P.G.; Shin, J.S.; Dige, N.C.; Vanjare, B.D.; Han, Y.; Choi, N.G.; Kim, S.J.; Seo, S.Y.; Lee, K.H. Chelation enhanced fluorescence of rhodamine based novel organic nanoparticles for selective detection of mercury ions in aqueous medium and intracellular cell imaging. J. Photochem. Photobiol. A Chem. 2020, 397, 112579. [Google Scholar] [CrossRef]
- Pallares, R.M.; Carter, K.P.; Zeltmann, S.E.; Tratnjek, T.; Minor, A.M.; Abergel, R.J. Selective Lanthanide Sensing with Gold Nanoparticles and Hydroxypyridinone Chelators. Inorg. Chem. 2020, 59, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Mondal, T.K.; Mondal, S.; Ghorai, U.K.; Saha, S.K. White light emitting lanthanide based carbon quantum dots as toxic Cr (VI) and pH sensor. J. Colloid Interface Sci. 2019, 553, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Shi, L.; Zhang, Y.; Zhang, G.; Zhang, C.; Dong, C.; Shuang, S. Smilax China-derived yellow-fluorescent carbon dots for temperature sensing, Cu2+ detection and cell imaging. Analyst 2020, 145, 2176–2183. [Google Scholar] [CrossRef]
- Du, F.; Li, G.; Gong, X.; Zhonghui, G.; Shuang, S.; Xian, M.; Dong, C. Facile, rapid synthesis of N,P-dual-doped carbon dots as a label-free multifunctional nanosensor for Mn(VII) detection, temperature sensing and cellular imaging. Sens. Actuators B Chem. 2018, 277, 492–501. [Google Scholar] [CrossRef]
- He, J.; He, Y.; Chen, Y.; Lei, B.; Zhuang, J.; Xiao, Y.; Liang, Y.; Zheng, M.; Zhang, H.; Liu, Y. Solid-State Carbon Dots with Red Fluorescence and Efficient Construction of Dual-Fluorescence Morphologies. Small 2017, 13, 1700075. [Google Scholar] [CrossRef] [PubMed]
- Cerõn, E.N.; Ortgies, D.; del Rosal, B.; Ren, F.; Benayas, A.; Vetrone, F.; Ma, D.; Sanz-Rodríguez, F.; Solé, J.G.; Jaque, D.; et al. Hybrid Nanostructures for High-Sensitivity Luminescence Nanothermometry in the Second Biological Window. Adv. Mater. 2015, 27, 4781–4787. [Google Scholar] [CrossRef]
- Peng, H.; Stich, M.I.J.; Yu, J.; Sun, L.-N.; Fischer, L.H.; Wolfbeis, O.S. Luminescent europium(III) nanoparticles for sensing and imaging of temperature in the physiological range. Adv. Mater. 2010, 22, 716–719. [Google Scholar] [CrossRef]
- Zairov, R.; Shamsutdinova, N.; Podyachev, S.; Sudakova, S.; Gimazetdinova, G.; Rizvanov, I.; Syakaev, V.; Babaev, V.; Amirov, R.; Mustafina, A. Structure impact in antenna effect of novel upper rim substituted tetra-1,3-diketone calix[4]arenes on Tb(III) green and Yb(III) NIR-luminescence. Tetrahedron 2016, 72, 2447–2455. [Google Scholar] [CrossRef]
- Du, F.; Zeng, F.; Ming, Y.; Wu, S. Carbon dots-based fluorescent probes for sensitive and selective detection of iodide. Microchim. Acta 2013, 180, 453–460. [Google Scholar] [CrossRef]
- Kalytchuk, S.; Poláková, K.; Wang, Y.; Froning, J.P.; Cepe, K.; Rogach, A.L.; Zbořil, R. Carbon Dot Nanothermometry: Intracellular Photoluminescence Lifetime Thermal Sensing. ACS Nano 2017, 11, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Martín, E.; Martínez, L.; Haro, P. Fluorescent nanothermometers for intracellular thermal sensing. Nanomedicine 2014, 9, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Yan, L.; Xu, H.; Yue, M. One-step synthesis of multi-emission carbon nanodots for ratiometric temperature sensing. Appl. Surf. Sci. 2018, 427, 1118–1123. [Google Scholar] [CrossRef]
- Wang, C.; Lin, H.; Xu, Z.; Huang, Y.; Humphrey, M.G.; Zhang, C. Tunable Carbon-Dot-Based Dual-Emission Fluorescent Nanohybrids for Ratiometric Optical Thermometry in Living Cells. ACS Appl. Mater. Interfaces 2016, 8, 6621–6628. [Google Scholar] [CrossRef]
- Macairan, J.R.; Jaunky, D.B.; Piekny, A.; Naccache, R. Intracellular ratiometric temperature sensing using fluorescent carbon dots. Nanoscale Adv. 2019, 1, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Zairov, R.R.; Dovzhenko, A.P.; Sapunova, A.S.; Voloshina, A.D.; Sarkanich, K.A.; Daminova, A.G.; Nizameev, I.R.; Lapaev, D.V.; Sudakova, S.N.; Podyachev, S.N.; et al. Terbium(III)-thiacalix[4]arene nanosensor for highly sensitive intracellular monitoring of temperature changes within the 303–313 K range. Sci. Rep. 2020, 10, 20541. [Google Scholar] [CrossRef]
- Shamsutdinova, N.; Zairov, R.; Nizameev, I.; Gubaidullin, A.; Mukhametshina, A.; Podyachev, S.; Ismayev, I.; Kadirov, M.; Voloshina, A.; Mukhametzyanov, T.; et al. Tuning magnetic relaxation properties of “hard cores” in core-shell colloids by modification of “soft shell”. Colloids Surf. B Biointerfaces 2018, 162, 52–59. [Google Scholar] [CrossRef] [PubMed]









| Name | d, nm | PDI | Zp, mV |
|---|---|---|---|
| PSS[TbL] | 193.3 ± 1.2 | 0.171 ± 0.011 | −26.2 ± 0.6 |
| PSS-{CDs-[TbL]} | 285.2 ± 1.2 | 0.258 ± 0.015 | −45.7 ± 0.7 |
| CDs | 1648 ± 523.1 | 0.951 ± 0.043 | −39.9 ± 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zairov, R.R.; Dovzhenko, A.P.; Sarkanich, K.A.; Nizameev, I.R.; Luzhetskiy, A.V.; Sudakova, S.N.; Podyachev, S.N.; Burilov, V.A.; Vatsouro, I.M.; Vomiero, A.; et al. Single Excited Dual Band Luminescent Hybrid Carbon Dots-Terbium Chelate Nanothermometer. Nanomaterials 2021, 11, 3080. https://doi.org/10.3390/nano11113080
Zairov RR, Dovzhenko AP, Sarkanich KA, Nizameev IR, Luzhetskiy AV, Sudakova SN, Podyachev SN, Burilov VA, Vatsouro IM, Vomiero A, et al. Single Excited Dual Band Luminescent Hybrid Carbon Dots-Terbium Chelate Nanothermometer. Nanomaterials. 2021; 11(11):3080. https://doi.org/10.3390/nano11113080
Chicago/Turabian StyleZairov, Rustem R., Alexey P. Dovzhenko, Kirill A. Sarkanich, Irek R. Nizameev, Andrey V. Luzhetskiy, Svetlana N. Sudakova, Sergey N. Podyachev, Vladimir A. Burilov, Ivan M. Vatsouro, Alberto Vomiero, and et al. 2021. "Single Excited Dual Band Luminescent Hybrid Carbon Dots-Terbium Chelate Nanothermometer" Nanomaterials 11, no. 11: 3080. https://doi.org/10.3390/nano11113080