Polychlorinated Biphenyl Profile in Polyhydroxy-alkanoates Synthetized from Urban Organic Wastes
Abstract
:1. Introduction
2. Experimental
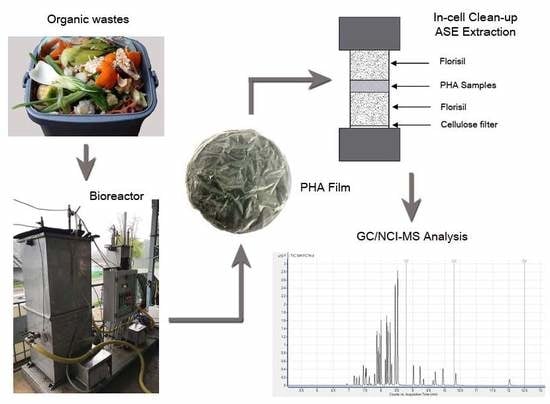
2.1. PHA Production and Extraction
2.2. PHA Characterization
2.3. Sample Preparation
2.4. GC–MS Analysis
2.5. Calibration Curves
3. Results and Discussion
3.1. Preliminary Assessment of PHA Composition, and Td
3.2. Method Performance
3.3. PCB Concentrations in PHA Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fidalgo-Used, N.; Blanco-Gonzalez, E.; Sanz-Medel, A. Sample handling strategies for the determination of persistent trace organic contaminants from biota samples. Anal. Chim. Acta 2007, 590, 1–16. [Google Scholar] [CrossRef]
- IARC-International Agency for Research on Cancer. Polychlorinated and Polybrominated Biphenyls; IARC Monographs: Lyon, France, 2016; Volume 107.
- Davodi, M.; Esmaili-Sari, A.; Bahramifarr, N. Concentration of polychlorinated biphenyls and organochlorine pesticides in some edible fish species from the Shadegan Marshes (Iran). Ecotoxicol. Environ. Saf. 2011, 74, 294–300. [Google Scholar] [CrossRef]
- Stockholm Convention. Stockholm Convention on Persistent Organic Pollutants (POPs), in: Stockholm Convention Persistent Organic Pollutants, 64. 2009. Available online: http://www.pops.int/TheConvention/Overview/TextoftheConvention/tabid/2232/Default.aspx (accessed on 2 February 2020).
- ICES-International Council for the Exploration of the Sea. Determination of Polychlorinatedbiphenyls (PCBs) in Sediment and Biota; Techniques in Marine Environmental Sciences: Copenhagen, Denmark, 2013; Volume 53, pp. 1–18. [Google Scholar]
- Reddy, A.V.B.; Moniruzzaman, M.; Aminabhavi, T.M. Polychlorinated biphenyls (PCBs) in the environment: Recent updates on sampling, pretreatment, cleanup technologies and their analysis. Chem. Eng. J. 2019, 358, 1186–1207. [Google Scholar] [CrossRef]
- Hu, D.; Hornbuckle, K.C. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 2010, 44, 2822–2827. [Google Scholar] [CrossRef] [PubMed]
- Tue, N.M.; Takahashi, S.; Suzuki, G.; Isobe, T.; Viet, P.H.; Kobara, Y.; Seike, N.; Zhang, G.; Sudaryanto, A.; Tanabe, S. Contamination of indoor dust and air by polychlorinated biphenyls and brominated flame retardants and relevance of non-dietary exposure in Vietnamese informal e-waste recycling sites. Environ. Int. 2013, 51, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Pomata, D.; Di Filippo, P.; Riccardi, C.; Rossi, V.; Simonetti, G.; Sonego, E.; Buiarelli, F. Method optimization for the simultaneous determination of legacy and emerging halogenated flame retardants in particulate matter collected in an electronic waste recycling facility. Int. J. Environ. Anal. Chem. 2019, 1–18. [Google Scholar] [CrossRef]
- Sakai, S.; Hayakawa, K.; Takatsuki, H.; Kawakami, I. Dioxin-like PCB released from waste incineration and their deposition flux. Environ. Sci. Technol. 2001, 35, 3601–3607. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Noma, Y.; Mori, Y.; Sakai, S. Congener profiles of PCB and a proposed new set of indicator congeners. Chemosphere 2007, 67, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Noma, Y.; Ishikawa, Y.; Nose, K.; Minetomatsu, K.; Takigami, H.; Sakai, S. Chemical characterization of PCB and dioxins in the waste PCB stockpiles. J. Environ. Chem. 2004, 14, 501–518. [Google Scholar] [CrossRef] [Green Version]
- Srogi, K. Levels and congener distributions of PCDDs, PCDFs and dioxin-like PCBs in environmental and human samples: A review. Environ. Chem. Lett. 2008, 6, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Cetin, B. Investigation of PAHs, PCBs and PCNs in soils around a heavily industrialized Area in Kocaeli, Turkey: Concentrations, distributions, sources and toxicological effects. Sci. Total Environ. 2016, 560–561, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Nilsson, M.L.; Kylin, H. Current-use and organochlorine pesticides and polychlorinated biphenyls in the biodegradable fraction of source separated household waste, compost, and anaerobic digestate. Bull. Environ. Contam. Toxicol. 2011, 86, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barret, M.; Carrere, H.; Latrille, E.; Wisniewski, C.; Patureau, D. Micropollutant and sludge characterization for modeling sorption equilibria. Environ. Sci. Technol. 2010, 44, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- COM. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: Closing the Loop—An EU Action Plan for the Circular Economy. 2015. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52015DC0614 (accessed on 2 February 2020).
- Saveyn, H.; Eder, P. End-of-Waste Criteria for Biodegradable Waste Subjected to Biological Treatment (Compost & Digestate): Technical Proposals. 2014. Available online: http://publications.jrc.ec.europa.eu/repository/handle/JRC87124 (accessed on 2 February 2020).
- Valentino, F.; Riccardi, C.; Campanari, S.; Pomata, D.; Majone, M. Fate of b-hexachlorocyclohexane in the mixed microbial cultures (MMCs) three-stage polyhydroxyalkanoates (PHA) production process from cheese whey. Bioresour. Technol. 2015, 192, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.B.S.; Malafaia, C.B. Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int. J. Boil. Macromol. 2018, 107, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Noor, S.; Khalil, S. Recent developments in the synthesis of poly(hydroxybutyrate) based biocomposites. Biotechnol. Prog. 2019, 35, e2855. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, J.K.; Chandel, A.K.; Sharma, L.; Mallick, N.; Singh, S.P. Biomedical applications of microbially engineered polyhydroxyalkanoates: An insight into recent advances, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2019, 103, 2007–2032. [Google Scholar] [CrossRef]
- Ghosh, R.; Hageman, K.J.; Björklund, E. Selective pressurized liquid extraction of three classes of halogenated contaminants in fish. J. Chromatogr. A 2011, 1218, 7242–7247. [Google Scholar] [CrossRef]
- Zhang, Z.; Ohiozebau, E.; Rhind, S.M. Simultaneous extraction and clean-up of polybrominated diphenyl ethers and polychlorinated biphenyls from sheep liver tissue by selective pressurized liquid extraction and analysis by gas chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 1203–1209. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. In-cell clean-up pressurized liquid extraction and gaschromatography–tandem mass spectrometry determination of hydrophobic persistent and emerging organic pollutants in coastal sediments. J. Chromatogr. A 2016, 1429, 107–118. [Google Scholar] [CrossRef]
- Valentino, F.; Moretto, G.; Lorini, L.; Bolzonella, D.; Pavan, P.; Majone, M. Pilot scale polyhydroxyalkanoate (PHA) production from combined treatment of organic fraction of municipal solid waste and sewage sludge. Ind. Eng. Chem. Res. 2019, 58, 12149–12158. [Google Scholar] [CrossRef]
- Moretto, G.; Russo, I.; Bolzonella, D.; Pavan, P.; Majone, M.; Valentino, F. An urban biorefinery for food waste and biological sludge conversion into polyhydroxyalkanoates and biogas. Water Res. 2020, 170, 115371. [Google Scholar] [CrossRef] [PubMed]
- Braunegg, G.; Sonnleitner, B.; Lafferty, R.M. A rapid gas chromatographic method for the determination of poly-?-hydroxybutyric acid in microbial biomass. Appl. Microbiol. Biotechnol. 1978, 6, 29–37. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Okamura, K.; Su, C.J. Physical Properties of Poly (β-hydroxybutyrate). II. Conformational Aspects in Solution. Macromolecules 1970, 3, 735–740. [Google Scholar] [CrossRef]
- Pena-Abaurrea, M.; Ramos, J.J.; Gonzalez, M.J.; Ramos, L. Miniaturized selective pressurized liquid extraction of polychlorinated biphenyls and polybrominated diphenyl ethers from feedstuffs. J. Chromatogr. A 2013, 1273, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Wang, X.; Cai, Z. Analytical chemistry of the persistent organic pollutants identified in the Stockholm Convention: A review. Anal. Chim. Acta 2013, 790, 1–13. [Google Scholar] [CrossRef]
- EPA. Polychlorinated Biphenyls (PCBs) by Gas Chromatography. Method 8082A, 1–56; 2007. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/8082a.pdf (accessed on 4 February 2020).
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- AOAC International. Guidelines for Standard Method Performance Requirements (Appendix F); AOAC Official Methods Analysis; AOAC International: Rockville, MD, USA, 2016; pp. 1–18. [Google Scholar]
- Cavaliere, C.; Montone, C.M.; Capriotti, A.L.; La Barbera, G.; Piovesana, S.; Rotatori, M.; Valentino, F.; Laganà, A. Extraction of polycyclic aromatic hydrocarbons from polyhydroxyalkanoates before gas chromatography/mass spectrometry analysis. Talanta 2018, 188, 671–675. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology 2014, Q2(R1), 1–13. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 4 February 2020).
- Abdallah, M.A.E.; Drageb, D.; Harrad, S. A one-step extraction/clean-up method for determination of PCBs, PBDEs and HBCDs in environmental solid matrices. Environ. Sci. Proc. Impacts 2013, 15, 2279–2287. [Google Scholar] [CrossRef]
- Perez, R.A.; Tadeo, J.L.; Albero, B.; Miguel, E.; Sánchez-Brunete, C. Gas chromatography-triple-quadrupole mass spectrometry for analysis of selected polyhalogenated pollutants in plants. Comparison of extraction methods. Anal. Bioanal. Chem. 2013, 405, 389–400. [Google Scholar] [CrossRef]
- FDA. 1996 Unavoidable Contaminants in Food for Human Consumption Food Packaging Material: Tolerances for Polychlorinated Biphenyls (PCBs), U.S. Food and Drug Administration 21 CFR 10930. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=109.30 (accessed on 4 February 2020).
- EC Regulation No. 1881/2006. Setting maximum levels contaminants in foodstuffs. Off. J. Eur. Union. 2006, L364, 5–24. [Google Scholar]
- EPA. Polychlorinated Biphenyls (PCBs); Recycling Plastics from Shredder Residue. Federal Register, Vol. 78, No. 66, Notices; 2013. Available online: https://www.federalregister.gov/documents/2013/04/05/2013-07981/polychlorinated-biphenyls-pcbs-recycling-plastics-from-shredder-residue (accessed on 4 February 2020).
- Oskam, I.C.; Ropstad, E.; Smith, A.J.; Skaare, J.U.; Tverdal, A.; Berg, K.A.; Wigerd, R. Effects of PCB99 and PCB153 exposure on spermatogenesis in young adult C57BL6 mice. Reprod. Toxicol. 2004, 19, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Maštovská, K.; Lehotay, S.J. Evaluation of analyte protectants to improve gaschromatographic analysis of pesticides. J. Chromatogr. A 2003, 1015, 163–184. [Google Scholar] [CrossRef]
- Hao, C.; Zhao, X.; Yang, P. GC-MS and HPLC-MS analysis of bioactive pharmaceutical sand personal-care products in environmental matrices. Trends Anal. Chem. 2007, 26, 569–580. [Google Scholar] [CrossRef]
- Rimayi, C.; Odusanya, D.; Mtunzi, F.; Tsoka, S. Alternative calibration techniques for counteracting the matrix effects in GC–MS-SPE pesticide residue analysis—A statistical approach. Chemosphere 2015, 118, 35–43. [Google Scholar] [CrossRef]
- Buiarelli, F.; Di Filippo, P.; Pomata, D.; Riccardi, C.; Bartocci, M. A liquid chromatography tandem mass spectrometry method for simultaneous analysis of 46 atmospheric particulate-phase persistent organic pollutants and comparison with gas chromatography/mass spectrometry. Int. J. Environ. Anal. Chem. 2017, 97, 797–818. [Google Scholar] [CrossRef]
- Salgueiro-González, N.; Turnes-Carou, I.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Analysis of endocrine disruptor compounds in marine sediments by in cell clean up-pressurized liquid extraction-liquid chromatography tandem mass spectrometry determination. Anal. Chim. Acta 2014, 852, 112–120. [Google Scholar] [CrossRef]
| Compound | LOD (μg·kg−1) | LOQ (μg·kg−1) | RSD Intra-Day | RSD Inter-Day |
|---|---|---|---|---|
| PCB28 | 3 | 11 | 1.8 | 3 |
| PCB77 | 0.12 | 0.42 | 1.1 | 2 |
| PCB81 | 0.14 | 0.47 | 6.9 | 15 |
| PCB99 | 9.8 | 30 | 1.8 | 14 |
| PCB101 | 0.09 | 0.3 | 8.1 | 11 |
| PCB105 | 0.06 | 0.2 | 12 | 19 |
| PCB110 | 0.17 | 0.56 | 11 | 20 |
| PCB114 | 0.08 | 0.27 | 4.8 | 9 |
| PCB126 | 0.12 | 0.41 | 2.8 | 10 |
| PCB138 | 0.11 | 0.37 | 8.4 | 15 |
| PCB146 | 0.10 | 0.35 | 3.6 | 17 |
| PCB151 | 0.08 | 0.27 | 4.8 | 8 |
| PCB156 | 0.13 | 0.42 | 2.2 | 5 |
| PCB157 | 0.11 | 0.37 | 2 | 5 |
| PCB167 | 0.12 | 0.40 | 0.9 | 6 |
| PCB169 | 0.12 | 0.39 | 0.6 | 3 |
| PCB(170 + 190) | 0.46 | 1.55 | 0.8 | 18 |
| PCB177 | 0.11 | 0.38 | 3.9 | 6 |
| PCB180 | 0.10 | 0.34 | 1 | 8 |
| PCB183 | 0.13 | 0.43 | 1.8 | 13 |
| PCB187 | 0.09 | 0.31 | 5.7 | 14 |
| Compound | Recovery (%) | ME (%) |
|---|---|---|
| PCB28 | 89 | 44 |
| PCB77 | 95 | 10 |
| PCB81 | 92 | 126 |
| PCB99 | 90 | 176 |
| PCB101 | 91 | 126 |
| PCB105 | 102 | 138 |
| PCB110 | 92 | 10 |
| PCB114 | 97 | 130 |
| PCB126 | 97 | 139 |
| PCB138 | 98 | 114 |
| PCB146 | 94 | 127 |
| PCB151 | 84 | 119 |
| PCB156 | 100 | 141 |
| PCB157 | 94 | 131 |
| PCB167 | 98 | 137 |
| PCB169 | 99 | 142 |
| PCB (170 + 190) | 97 | 137 |
| PCB177 | 97 | 134 |
| PCB180 | 97 | 136 |
| PCB183 | 94 | 131 |
| PCB187 | 97 | 135 |
| Extraction Method | ||||
|---|---|---|---|---|
| Raw PHA-Rich Biomass | NaClO | CHCl3 | Biotrend | |
| PCB28 | nd | nd | nd | nd |
| PCB77 | nd | 2.3 ± 0.0050 | 1.8 ± 0.0039 | 1.5 ± 0.046 |
| PCB81 | 1.7 ± 0.029 | 1.6 ± 0.030 | 2.6 ± 0.031 | nd |
| PCB99 | 47.3 ± 3.40 | 51 ± 2.6 | 48 ± 6.2 | nd |
| PCB101 | 2.6 ± 0.82 | 4.9 ± 2.3 | 2.4 ± 1.3 | 3.4 ± 0.020 |
| PCB105 | 1 ± 0.1 | 1.2 ± 0.040 | 1.3 ± 0.20 | 1.7 ± 0.029 |
| PCB110 | nd | nd | nd | nd |
| PCB114 | 1 ± 0.07 | 1 ± 0.06 | 1 ± 0.08 | 1.3 ± 0.013 |
| PCB126 | nd | nd | nd | 1.7 ± 0.012 |
| PCB138 | 2.7 ± 1.1 | 2.2 ± 0.48 | 2.9 ± 0.89 | 2.1 ± 0.89 |
| PCB146 | 1.3 ± 0.010 | 1.4 ± 0.050 | 1.4 ± 0.051 | 1.6 ± 0.031 |
| PCB151 | 0.90 ± 0.014 | nd | nd | 1.4 ± 0.010 |
| PCB156 | nd | nd | nd | 2 ± 0.02 |
| PCB157 | nd | nd | nd | 1.7 ± 0.011 |
| PCB167 | nd | nd | nd | 1.7 ± 0.0039 |
| PCB169 | 3.4 ± 0.70 | 1.7 ± 0.20 | 1.6 ± 0.13 | 1.9 ± 0.30 |
| PCB (170 + 190) | nd | 6.6 ± 0.020 | nd | 7.1 ± 0.010 |
| PCB177 | 1.4 ± 0.079 | 1.5 ± 0.10 | 1.5 ± 0.10 | 1.6 ± 0.69 |
| PCB180 | 1.8 ± 0.29 | 1.6 ± 0.20 | 1.7 ± 0.14 | 1.8 ± 0.0040 |
| PCB183 | 1.4 ± 0.011 | 1.5 ± 0.050 | 1.5 ± 0.047 | 1.9 ± 0.012 |
| PCB187 | 1.6 ± 0.25 | 1.4 ± 0.018 | 1.5 ± 0.050 | 1.5 ± 0.019 |
| Fruit Waste | Commercial | |
|---|---|---|
| PCB28 | nd | nd |
| PCB77 | nd | 2 ± 0.005 |
| PCB81 | nd | 2.3 ± 0.040 |
| PCB99 | nd | 50 ± 2.6 |
| PCB101 | nd | 2.7 ± 0.031 |
| PCB105 | nd | 1.2 ± 0.020 |
| PCB110 | nd | nd |
| PCB114 | nd | 1.4 ± 0.019 |
| PCB126 | nd | 1.5 ± 0.30 |
| PCB138 | 1.4 ± 0.21 | 2.1 ± 0.35 |
| PCB146 | nd | 1.5 ± 0.019 |
| PCB151 | nd | 1.1 ± 0.10 |
| PCB156 | nd | 1.9 ± 0.011 |
| PCB157 | nd | 1.7 ± 0.0048 |
| PCB167 | nd | 1.8 ± 0.029 |
| PCB169 | 2.3 ± 0.45 | 1.7 ± 0.17 |
| PCB (170 + 190) | 8.5 ± 0.012 | nd |
| PCB177 | nd | 1.7 ± 0.010 |
| PCB180 | nd | 1.5 ± 0.10 |
| PCB183 | nd | 1.8 ± 0.0057 |
| PCB187 | nd | 1.4 ± 0.050 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccardi, C.; Buiarelli, F.; Castellani, F.; Di Filippo, P.; Lorini, L.; Majone, M.; Matos, M.; Pomata, D.; Simonetti, G.; Sommer Ferreira, B.; et al. Polychlorinated Biphenyl Profile in Polyhydroxy-alkanoates Synthetized from Urban Organic Wastes. Polymers 2020, 12, 659. https://doi.org/10.3390/polym12030659
Riccardi C, Buiarelli F, Castellani F, Di Filippo P, Lorini L, Majone M, Matos M, Pomata D, Simonetti G, Sommer Ferreira B, et al. Polychlorinated Biphenyl Profile in Polyhydroxy-alkanoates Synthetized from Urban Organic Wastes. Polymers. 2020; 12(3):659. https://doi.org/10.3390/polym12030659
Chicago/Turabian StyleRiccardi, Carmela, Francesca Buiarelli, Federica Castellani, Patrizia Di Filippo, Laura Lorini, Mauro Majone, Mariana Matos, Donatella Pomata, Giulia Simonetti, Bruno Sommer Ferreira, and et al. 2020. "Polychlorinated Biphenyl Profile in Polyhydroxy-alkanoates Synthetized from Urban Organic Wastes" Polymers 12, no. 3: 659. https://doi.org/10.3390/polym12030659